Cytolytic CD8+ T cells recognizing CFP10 are recruited to the lung after Mycobacterium tuberculosis infection
- PMID: 15557351
- PMCID: PMC2211947
- DOI: 10.1084/jem.20041690
Cytolytic CD8+ T cells recognizing CFP10 are recruited to the lung after Mycobacterium tuberculosis infection
Abstract
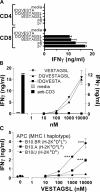
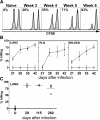
Optimum immunity against Mycobacterium tuberculosis requires both CD4(+) and CD8(+) T cells. In contrast with CD4(+) T cells, few antigens are known that elicit CD8(+) T cells during infection. CD8(+) T cells specific for culture filtrate protein-10 (CFP10) are found in purified protein derivative positive donors, suggesting that CFP10 primes CD8(+) T cells in vivo. Using T cells from M. tuberculosis-infected mice, we identified CFP10 epitopes recognized by CD8(+) T cells and CD4(+) T cells. CFP10-specific T cells were detected as early as week 3 after infection and at their peak accounted for up to 30% of CD8(+) T cells in the lung. IFNgamma-producing CD8(+) and CD4(+) T cells recognizing CFP10 epitopes were preferentially recruited to the lungs of M. tuberculosis-infected mice. In vivo cytolytic activity of CD8(+) T cells specific for CFP10 and TB10.3/10.4 proteins was detected in the spleen, pulmonary lymph nodes, and lungs of infected mice. The cytolytic activity persisted long term and could be detected 260 d after infection. This paper highlights the cytolytic function of antigen-specific CD8(+) T cells elicited by M. tuberculosis infection and demonstrates that large numbers of CFP10-specific cytolytic CD8(+) T cells are recruited to the lung after M. tuberculosis infection.
Figures




References
-
- Flynn, J.L., and J. Chan. 2001. Immunology of tuberculosis. Annu. Rev. Immunol. 19:93–129. - PubMed
-
- Smith, S.M., and H.M. Dockrell. 2000. Role of CD8 T cells in mycobacterial infections. Immunol. Cell Biol. 78:325–333. - PubMed
-
- Ackerman, A.L., and P. Cresswell. 2004. Cellular mechanisms governing cross-presentation of exogenous antigens. Nat. Immunol. 5:678–684. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

