Subcellular distribution of enzyme I of the Escherichia coli phosphoenolpyruvate:glycose phosphotransferase system depends on growth conditions
- PMID: 15557553
- PMCID: PMC536035
- DOI: 10.1073/pnas.0407865101
Subcellular distribution of enzyme I of the Escherichia coli phosphoenolpyruvate:glycose phosphotransferase system depends on growth conditions
Abstract
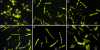
The phosphoenolpyruvate:glycose phosphotransferase system (PTS) participates in important functions in the bacterial cell, including the phosphorylation/uptake of PTS sugars. Enzyme I (EI), the first protein of the PTS complex, accepts the phosphoryl group from phosphoenolpyruvate, which is then transferred through a chain of proteins to the sugar. In these studies, a mutant GFP, enhanced yellow fluorescent protein (YFP), was linked to the N terminus of EI, giving Y-EI. Y-EI was active both in vitro (>/=90% compared with EI) and in vivo. Unexpectedly, the subcellular distribution of Y-EI varied significantly. Three types of fluorescence were observed: (i) diffuse (dispersed throughout the cell), (ii) punctate (concentrated in numerous discrete spots throughout the cell), and (iii) polar (at one or both ends of the cell). Cells from dense colonies grown on agar plates with LB broth or synthetic (Neidhardt) medium showed primarily bipolar or punctate fluorescence. In liquid culture, under carefully defined carbon-limiting growth conditions [ribose (non-PTS), mannitol (PTS sugar), or dl-lactate], cellular levels of enzymatically active Y-EI remain essentially constant for each carbon source, but fluorescence distribution depends on C source, cell density, growth phase, and apparently on "conditioned medium." Fluorescence was diffuse during exponential growth on LB or ribose/Neidhardt medium. On ribose they became punctate in the stationary phase, reverting to diffuse when more ribose was added. In LB, both Y-EI and a nonphosphorylatable mutant, H189Q-Y-EI, showed a diffuse fluorescence during growth, but, shortly after the addition of isopropyl beta-d-thiogalactopyranoside, Y-EI became bipolar; H189Q-Y-EI did not. The functions of EI sequestration remain to be determined.
Figures




Similar articles
-
The monomer/dimer transition of enzyme I of the Escherichia coli phosphotransferase system.J Biol Chem. 2006 Jun 30;281(26):17570-8. doi: 10.1074/jbc.M508965200. Epub 2006 Mar 19. J Biol Chem. 2006. PMID: 16547355
-
Properties of the C-terminal domain of enzyme I of the Escherichia coli phosphotransferase system.J Biol Chem. 2006 Jun 30;281(26):17579-87. doi: 10.1074/jbc.M508966200. Epub 2006 Mar 19. J Biol Chem. 2006. PMID: 16547354
-
The N-terminal domain of Escherichia coli enzyme I of the phosphoenolpyruvate/glycose phosphotransferase system: molecular cloning and characterization.Proc Natl Acad Sci U S A. 1996 Jul 9;93(14):7028-31. doi: 10.1073/pnas.93.14.7028. Proc Natl Acad Sci U S A. 1996. PMID: 8692938 Free PMC article.
-
Current knowledge of the Escherichia coli phosphoenolpyruvate-carbohydrate phosphotransferase system: peculiarities of regulation and impact on growth and product formation.Appl Microbiol Biotechnol. 2012 Jun;94(6):1483-94. doi: 10.1007/s00253-012-4101-5. Epub 2012 May 11. Appl Microbiol Biotechnol. 2012. PMID: 22573269 Review.
-
Carbohydrate Transport by Group Translocation: The Bacterial Phosphoenolpyruvate: Sugar Phosphotransferase System.Subcell Biochem. 2019;92:223-274. doi: 10.1007/978-3-030-18768-2_8. Subcell Biochem. 2019. PMID: 31214989 Review.
Cited by
-
Streptococcus pyogenes polymyxin B-resistant mutants display enhanced ExPortal integrity.J Bacteriol. 2014 Jul;196(14):2563-77. doi: 10.1128/JB.01596-14. Epub 2014 May 2. J Bacteriol. 2014. PMID: 24794568 Free PMC article.
-
Dynamic localization of a transcription factor in Bacillus subtilis: the LicT antiterminator relocalizes in response to inducer availability.J Bacteriol. 2013 May;195(10):2146-54. doi: 10.1128/JB.00117-13. Epub 2013 Mar 8. J Bacteriol. 2013. PMID: 23475962 Free PMC article.
-
The histone deacetylase Hos2 forms an Hsp42-dependent cytoplasmic granule in quiescent yeast cells.Mol Biol Cell. 2012 Apr;23(7):1231-42. doi: 10.1091/mbc.E11-09-0752. Epub 2012 Feb 15. Mol Biol Cell. 2012. PMID: 22337769 Free PMC article.
-
Functional taxonomy of bacterial hyperstructures.Microbiol Mol Biol Rev. 2007 Mar;71(1):230-53. doi: 10.1128/MMBR.00035-06. Microbiol Mol Biol Rev. 2007. PMID: 17347523 Free PMC article. Review.
-
The membrane: transertion as an organizing principle in membrane heterogeneity.Front Microbiol. 2015 Jun 12;6:572. doi: 10.3389/fmicb.2015.00572. eCollection 2015. Front Microbiol. 2015. PMID: 26124753 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

