Yersinia enterocolitica adhesin A induces production of interleukin-8 in epithelial cells
- PMID: 15557598
- PMCID: PMC529134
- DOI: 10.1128/IAI.72.12.6780-6789.2004
Yersinia enterocolitica adhesin A induces production of interleukin-8 in epithelial cells
Abstract
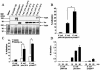
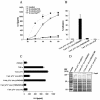
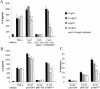
The major invasive factor of Yersinia enterocolitica, the invasin (Inv) protein, induces proinflammatory host cell responses, including interleukin-8 (IL-8) secretion from human epithelial cells, by engagement of beta1 integrins. The Inv-triggered beta1 integrin signaling involves the small GTPase Rac; the activation of MAP kinases, such as p38, MEK1, and JNK; and the activation of the transcription factor NF-kappaB. In the present study, we demonstrate that Y. enterocolitica YadA, which is a major adhesin of Y. enterocolitica with pleiotropic virulence effects, induces IL-8 secretion in epithelial cells. The abilities of YadA and Inv to promote adhesion to and invasion of HeLa cells and to induce IL-8 production by the cells were investigated by expression of YadA and Inv in Escherichia coli. While YadA mediates efficacious adhesion to HeLa cells, it mediates marginal invasion compared with Inv. Both YadA and Inv trigger comparable levels of IL-8 production. Conformational changes of the YadA head domain by mutation of NSVAIG-S motifs, which abolish collagen binding, also abolish adhesion of Yersinia to HeLa cells and YadA-mediated IL-8 secretion. Furthermore, experiments in which blocking antibodies against beta1 integrins were used demonstrate that beta1 integrins are crucial for YadA-mediated IL-8 secretion. Inhibitor studies demonstrate the involvement of small GTPases and MAP kinases, such as p38, MEK1, and JNK, indicating that beta1 integrin-dependent signaling mediated by Inv or YadA involves similar signaling pathways. These data present YadA, in addition to Inv, YopB, and Yersinia lipopolysaccharide, as a further inducer of proinflammatory molecules by which Y. enterocolitica might promote inflammatory tissue reactions.
Figures

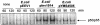
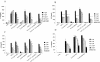
References
-
- Bohn, E., S. Muller, J. Lauber, R. Geffers, N. Speer, C. Spieth, J. Krejci, B. Manncke, J. Buer, A. Zell, and I. B. Autenrieth. 2004. Gene expression patterns of epithelial cells modulated by pathogenicity factors of Yersinia enterocolitica. Cell Microbiol. 6:129-141. - PubMed
-
- Bottone, E. J. 1977. Yersinia enterocolitica: a panoramic view of a charismatic microorganism. Crit. Rev. Microbiol. 5:211-241. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

