The mycobacterial heparin-binding hemagglutinin is a protective antigen in the mouse aerosol challenge model of tuberculosis
- PMID: 15557600
- PMCID: PMC529156
- DOI: 10.1128/IAI.72.12.6799-6805.2004
The mycobacterial heparin-binding hemagglutinin is a protective antigen in the mouse aerosol challenge model of tuberculosis
Abstract
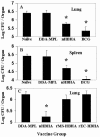
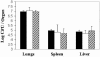
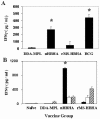
The heparin-binding hemagglutinin (HBHA) of Mycobacterium tuberculosis is a surface-expressed adhesin that can affect binding to host cells via a unique, methylated, carboxyl-terminal, lysine-, alanine-, and proline-rich repeat region. It has been implicated in extrapulmonary dissemination of M. tuberculosis from the lung following the initial infection of the host. To assess the vaccine potential of this protein, purified preparations of HBHA were emulsified in a dimethyldioctadecylammonium bromide-monophosphoryl lipid A adjuvant and tested for the ability to reduce M. tuberculosis infection in the mouse aerosol challenge model for tuberculosis. The HBHA-containing vaccine gave a approximately 0.7-log reduction in CFU in both mouse lungs and spleens compared to adjuvant controls 28 days following challenge. Although a notable level of serum antibody to HBHA was elicited after three immunizations and the antibodies were able to bind to the surface of M. tuberculosis, passive immunization with monoclonal antibodies directed against HBHA did not protect in the challenge model. Compared to adjuvant controls, an elevated gamma interferon response was generated by splenic and lymph node-derived T cells from immunized mice in the presence of macrophages pulsed with purified HBHA or infected with live M. tuberculosis, suggesting that the effective immunity may be cell mediated. Efforts to construct effective recombinant HBHA vaccines in fast-growing Mycobacterium smegmatis have been unsuccessful so far, which indicates that distinctive posttranslational modifications present in the HBHA protein expressed by M. tuberculosis are critical for generating effective host immune responses. The vaccine studies described here demonstrate that HBHA is a promising new vaccine candidate for tuberculosis.
Figures




Similar articles
-
Mucosal immunization with recombinant heparin-binding haemagglutinin adhesin suppresses extrapulmonary dissemination of Mycobacterium bovis bacillus Calmette-Guérin (BCG) in infected mice.Vaccine. 2008 Feb 13;26(7):924-32. doi: 10.1016/j.vaccine.2007.12.005. Epub 2007 Dec 26. Vaccine. 2008. PMID: 18192091
-
Heparin-binding hemagglutinin, from an extrapulmonary dissemination factor to a powerful diagnostic and protective antigen against tuberculosis.Tuberculosis (Edinb). 2006 May-Jul;86(3-4):303-9. doi: 10.1016/j.tube.2006.01.016. Epub 2006 Feb 28. Tuberculosis (Edinb). 2006. PMID: 16510310 Review.
-
[Novel vaccines against M. tuberculosis].Kekkaku. 2006 Dec;81(12):745-51. Kekkaku. 2006. PMID: 17240920 Review. Japanese.
-
Expression and purification of recombinant methylated HBHA in Mycobacterium smegmatis.FEMS Microbiol Lett. 2004 Oct 1;239(1):33-9. doi: 10.1016/j.femsle.2004.08.015. FEMS Microbiol Lett. 2004. PMID: 15451098
-
The heparin-binding haemagglutinin of M. tuberculosis is required for extrapulmonary dissemination.Nature. 2001 Jul 12;412(6843):190-4. doi: 10.1038/35084083. Nature. 2001. PMID: 11449276
Cited by
-
A Fluorescent Probe for Detecting Mycobacterium tuberculosis and Identifying Genes Critical for Cell Entry.Front Microbiol. 2016 Dec 20;7:2021. doi: 10.3389/fmicb.2016.02021. eCollection 2016. Front Microbiol. 2016. PMID: 28066347 Free PMC article.
-
Mycobacterium tuberculosis Dissemination Plays a Critical Role in Pathogenesis.Front Cell Infect Microbiol. 2020 Feb 25;10:65. doi: 10.3389/fcimb.2020.00065. eCollection 2020. Front Cell Infect Microbiol. 2020. PMID: 32161724 Free PMC article. Review.
-
The role of antibodies in tuberculosis diagnosis, prophylaxis and therapy: a review from the ESGMYC study group.Eur Respir Rev. 2022 Mar 9;31(163):210218. doi: 10.1183/16000617.0218-2021. Print 2022 Mar 31. Eur Respir Rev. 2022. PMID: 35264411 Free PMC article. Review.
-
Mycobacterial Heparin-binding Hemagglutinin Antigen Activates Inflammatory Responses through PI3-K/Akt, NF-κB, and MAPK Pathways.Immune Netw. 2011 Apr;11(2):123-33. doi: 10.4110/in.2011.11.2.123. Epub 2011 Apr 30. Immune Netw. 2011. PMID: 21637390 Free PMC article.
-
Simultaneous Immunization with Multiple Diverse Immunogens Alters Development of Antigen-Specific Antibody-Mediated Immunity.Vaccines (Basel). 2021 Aug 28;9(9):964. doi: 10.3390/vaccines9090964. Vaccines (Basel). 2021. PMID: 34579201 Free PMC article.
References
-
- Betts, J. C., P. T. Lukey, L. C. Robb, R. A. McAdam, and K. Duncan. 2002. Evaluation of a nutrient starvation model of Mycobacterium tuberculosis persistence by gene and protein expression profiling. Mol. Microbiol. 43:717-731. - PubMed
-
- Brennan, M. J. 2004. A new generation of tuberculosis vaccines, p. 177-182. In C. A. de Quadros (ed.), Vaccines—preventing disease & protecting health. Pan American Health Organization, Washington, D.C.
-
- Collins, F. M. 1985. Protection to mice afforded by BCG vaccines against an aerogenic challenge by three mycobacteria of decreasing virulence. Tubercle 66:267-276. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

