Hemozoin differentially regulates proinflammatory cytokine production in human immunodeficiency virus-seropositive and -seronegative women with placental malaria
- PMID: 15557625
- PMCID: PMC529128
- DOI: 10.1128/IAI.72.12.7022-7029.2004
Hemozoin differentially regulates proinflammatory cytokine production in human immunodeficiency virus-seropositive and -seronegative women with placental malaria
Abstract
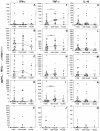
Pregnant women are at an increased risk for malarial infection. Plasmodium falciparum accumulates in the placenta and is associated with dysregulated immune function and poor birth outcomes. Malarial pigment (hemozoin) also accumulates in the placenta and may modulate local immune function. In this study, the impact of hemozoin on cytokine production by intervillous blood mononuclear cells from malaria-infected placentas was investigated. There was a dose-dependent, suppressive effect of hemozoin on production of gamma interferon (IFN-gamma), with less of an effect on tumor necrosis factor alpha (TNF-alpha) and interleukin-10, in human immunodeficiency virus-seronegative (HIV(-)) women. In contrast, IFN-gamma and TNF-alpha production tended to increase in HIV-seropositive women with increasing hemozoin levels. Production patterns of cytokines, especially IFN-gamma in HIV(-) women, followed different trends as a function of parasite density and hemozoin level. The findings suggest that the influences of hemozoin accumulation and high-density parasitemia on placental cytokine production are not equivalent and may involve different mechanisms, all of which may operate differently in the context of HIV infection. Cytokine production dysregulated by accumulation of hemozoin or high-density parasitemia may induce pathology and impair protective immunity in HIV-infected and -uninfected women.
Figures

References
-
- Arese, P., and E. Schwarzer. 1997. Malarial pigment (haemozoin): a very active ‘inert' substance. Ann. Trop. Med. Parasitol. 91:501-516. - PubMed
-
- Artavanis-Tsakonas, K., K. Eleme, K. L. McQueen, N. W. Cheng, P. Parham, D. M. Davis, and E. M. Riley. 2003. Activation of a subset of human NK cells upon contact with Plasmodium falciparum-infected erythrocytes. J. Immunol. 171:5396-5405. - PubMed
-
- Bouyou-Akotet, M. K., S. Issifou, J. F. Meye, M. Kombila, E. Ngou-Milama, A. J. Luty, P. G. Kremsner, and E. Mavoungou. 2004. Depressed natural killer cell cytotoxicity against Plasmodium falciparum-infected erythrocytes during first pregnancies. Clin. Infect. Dis. 38:342-347. - PubMed
-
- Bulmer, J. N., F. N. Rasheed, N. Francis, L. Morrison, and B. M. Greenwood. 1993. Placental malaria. I. Pathological classification. Histopathology 22:211-218. - PubMed
-
- Chaisavaneeyakorn, S., J. M. Moore, L. Mirel, C. Othoro, J. Otieno, S. C. Chaiyaroj, Y. P. Shi, B. L. Nahlen, A. A. Lal, and V. Udhayakumar. 2003. Levels of macrophage inflammatory protein 1α (MIP-1α) and MIP-1β in intervillous blood plasma samples from women with placental malaria and human immunodeficiency virus infection. Clin. Diagn. Lab. Immunol. 10:631-636. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

