A mutant of Mycobacterium tuberculosis H37Rv that lacks expression of antigen 85A is attenuated in mice but retains vaccinogenic potential
- PMID: 15557632
- PMCID: PMC529100
- DOI: 10.1128/IAI.72.12.7084-7095.2004
A mutant of Mycobacterium tuberculosis H37Rv that lacks expression of antigen 85A is attenuated in mice but retains vaccinogenic potential
Abstract
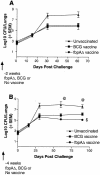
The fbpA and fbpB genes encoding the 85A and 85B proteins of Mycobacterium tuberculosis H37Rv, respectively, were disrupted, the mutants were examined for their ability to survive, and the strain lacking 85A (DeltafbpA) was tested for its ability to immunize mice. The DeltafbpA mutant was attenuated in mice after intravenous or aerosol infection, while replication of the DeltafbpB mutant was similar to that of the wild type. Complementation of the fbpA gene in DeltafbpA restored its ability to grow in the lungs of mice. The DeltafbpA mutant induced a stronger expression of pulmonary mRNA messages in mice for tumor necrosis factor alpha, interleukin-1 beta (IL-1beta), gamma interferon, IL-6, IL-2, and inducible nitric oxide (NO) synthase, which led to its decline, while H37Rv persisted despite strong immune responses. H37Rv and DeltafbpA both induced NO in macrophages and were equally susceptible to NO donors, although DeltafbpA was more susceptible in vitro to peroxynitrite and its growth was enhanced by NO inhibitors in mice and macrophages. Aerosol-infected mice, which cleared a low-dose DeltafbpA infection, resisted a challenge with virulent M. tuberculosis. Mice subcutaneously immunized with DeltafbpA or Mycobacterium bovis BCG and challenged with M. tuberculosis also showed similar levels of protection, marked by a reduction in the growth of challenged M. tuberculosis. The DeltafbpA mutant was thus attenuated, unlike DeltafbpB, but was also vaccinogenic against tuberculosis. Attenuation was incomplete, however, since DeltafbpA revived in normal mice after 370 days, suggesting that revival was due to immunosenescence but not compensation by the fbpB or fbpC gene. Antigen 85A thus affects susceptibility to peroxynitrite in M. tuberculosis and appears to be necessary for its optimal growth in mice.
Figures
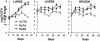






References
-
- Actor, J. K., T. Kuffner, C. S. Dezzutti, R. L. Hunter, and J. McNicholl. 1998. A flash-type bioluminescent immunoassay that is more sensitive than radioimaging: quantitative detection of cytokine cDNA in activated and resting human cells. J. Immunol. Methods 211:65-77. - PubMed
-
- Actor, J. K., M. Olsen, C. Jagannath, and R. L. Hunter. 1999. Relationship between survival, organism containment and granuloma formation in acute murine tuberculosis. J. Interferon Cytokine Res. 19:1183-1193. - PubMed
-
- Actor, J. K., E, Breij, H. Hoffmann, R. A. Wetsel, R. L. Hunter, and C. Jagannath. 2001. A role for complement C5 in organism containment and granulomatous response during murine tuberculosis in mice. Scand. J. Immunol. 53:464-474. - PubMed
-
- Armitige, L. Y., S. J. Norris, A. Wanger, T. R. Sievert, and K. Takayama. Submitted for publication.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

