Differential regulation of inflammatory cytokine secretion by human dendritic cells upon Chlamydia trachomatis infection
- PMID: 15557648
- PMCID: PMC529152
- DOI: 10.1128/IAI.72.12.7231-7239.2004
Differential regulation of inflammatory cytokine secretion by human dendritic cells upon Chlamydia trachomatis infection
Abstract
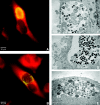
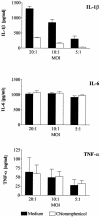
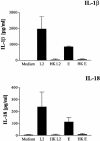
Chlamydia trachomatis is an obligate intracellular gram-negative bacterium responsible for a wide spectrum of diseases in humans. Both genital and ocular C. trachomatis infections are associated with tissue inflammation and pathology. Dendritic cells (DC) play an important role in both innate and adaptive immune responses to microbial pathogens and are a source of inflammatory cytokines. To determine the potential contribution of DC to the inflammatory process, human DC were infected with C. trachomatis serovar E or L2. Both C. trachomatis serovars were found to infect and replicate in DC. Upon infection, DC up-regulated the expression of costimulatory (B7-1) and cell adhesion (ICAM-1) molecules. Furthermore, chlamydial infection induced the secretion of interleukin-1beta (IL-1beta), IL-6, IL-8, IL-12p70, IL-18, and tumor necrosis factor alpha (TNF-alpha). The mechanisms involved in Chlamydia-induced IL-1beta and IL-18 secretion differed from those of the other cytokines. Chlamydia-induced IL-1beta and IL-18 secretion required infection with viable bacteria and was associated with the Chlamydia-induced activation of caspase-1 in infected host cells. In contrast, TNF-alpha and IL-6 secretion did not require that the Chlamydia be viable, suggesting that there are at least two mechanisms involved in the Chlamydia-induced cytokine secretion in DC. Interestingly, an antibody to Toll-like receptor 4 inhibited Chlamydia-induced IL-1beta, IL-6, and TNF-alpha secretion. The data herein demonstrate that DC can be infected by human C. trachomatis serovars and that chlamydial components regulate the secretion of various cytokines in DC. Collectively, these data suggest that DC play a role in the inflammatory processes caused by chlamydial infections.
Figures
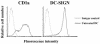
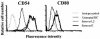


References
-
- Abu el-Asrar, A. M., K. Geboes, K. F. Tabbara, S. A. al Kharashi, L. Missotten, and V. Desmet. 1998. Immunopathogenesis of conjunctival scarring in trachoma. Eye 12(Pt. 3a):453-460. - PubMed
-
- Antonopoulos, C., M. Cumberbatch, R. J. Dearman, R. J. Daniel, I. Kimber, and R. W. Groves. 2001. Functional caspase-1 is required for Langerhans cell migration and optimal contact sensitization in mice. J. Immunol. 166:3672-3677. - PubMed
-
- Azuma, I., E. E. Ribi, T. J. Meyer, and B. Zbar. 1974. Biologically active components from mycobacterial cell walls. I. Isolation and composition of cell wall skeleton and component P3. J. Natl. Cancer Inst. 52:95-101. - PubMed
-
- Babcock, T. A., and J. M. Carlin. 2000. Transcriptional activation of indoleamine dioxygenase by interleukin 1 and tumor necrosis factor alpha in interferon-treated epithelial cells. Cytokine 12:588-594. - PubMed
-
- Bhardwaj, N. 2001. Processing and presentation of antigens by dendritic cells: implications for vaccines. Trends Mol. Med. 7:388-394. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

