Mannose-resistant Proteus-like fimbriae are produced by most Proteus mirabilis strains infecting the urinary tract, dictate the in vivo localization of bacteria, and contribute to biofilm formation
- PMID: 15557655
- PMCID: PMC529131
- DOI: 10.1128/IAI.72.12.7294-7305.2004
Mannose-resistant Proteus-like fimbriae are produced by most Proteus mirabilis strains infecting the urinary tract, dictate the in vivo localization of bacteria, and contribute to biofilm formation
Abstract
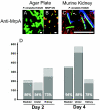
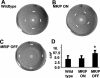
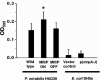
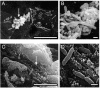
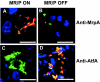
Proteus mirabilis, an etiologic agent of complicated urinary tract infections, expresses mannose-resistant Proteus-like (MR/P) fimbriae whose expression is phase variable. Here we examine the role of these fimbriae in biofilm formation and colonization of the urinary tract. The majority of wild-type P. mirabilis cells in transurethrally infected mice produced MR/P fimbriae. Mutants that were phase-locked for either constitutive expression (MR/P ON) or the inability to express MR/P fimbriae (MR/P OFF) were phenotypically distinct and swarmed at different rates. The number of P. mirabilis cells adhering to bladder tissue did not appear to be affected by MR/P fimbriation. However, the pattern of adherence to the bladder surface was strikingly different. MR/P OFF colonized the lamina propria underlying exfoliated uroepithelium, while MR/P ON colonized the luminal surfaces of bladder umbrella cells and not the exfoliated regions. Wild-type P. mirabilis was usually found colonizing intact uroepithelium, but it occasionally adhered to exfoliated areas. MR/P ON formed significantly more biofilm than either P. mirabilis HI4320 (P = 0.03) or MR/P OFF (P = 0.05). MR/P OFF was able to form a biofilm similar to that of the wild type. MR/P ON formed a three-dimensional biofilm structure as early as 18 h after the initiation of the biofilm, while MR/P OFF and the wild type did not. After 7 days, however, P. mirabilis HI4320 formed a 65-mum-thick biofilm, while the thickest MR/P ON and MR/P OFF biofilms were only 12 mum thick. We concluded that MR/P fimbriae are expressed by most P. mirabilis cells infecting the urinary tract, dictate the localization of bacteria in the bladder, and contribute to biofilm formation.
Figures




References
-
- Anderson, G. G., J. J. Palermo, J. D. Schilling, R. Roth, J. Heuser, and S. J. Hultgren. 2003. Intracellular bacterial biofilm-like pods in urinary tract infections. Science 301:105-107. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

