Treatment of bone pain secondary to metastases using samarium-153-EDTMP
- PMID: 15558143
- PMCID: PMC11160335
- DOI: 10.1590/s1516-31802004000500006
Treatment of bone pain secondary to metastases using samarium-153-EDTMP
Abstract
Context: More than 50% of patients with prostate, breast or lung cancer will develop painful bone metastases. The purpose of treating bone metastases is to relieve pain, reduce the use of steroids and to maintain motion.
Objective: To evaluate the use of samarium-153-EDTMP (153Sm-EDTMP) for the treatment of bone pain secondary to metastases that is refractory to clinical management.
Type of study: Retrospective.
Setting: Division of Nuclear Medicine, Universidade Estadual de Campinas (Unicamp).
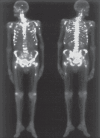
Methods: Fifty-eight patients were studied (34 males) with mean age 62 years; 31 patients had prostate cancer, 20 had breast cancer, three had lung cancer, one had lung hemangioendothelioma, one had parathyroid adenocarcinoma, one had osteosarcoma and one had an unknown primary tumor. All patients had multiple bone metastases demonstrated by bone scintigraphy using 99mTc-MDP,and were treated with 153Sm-EDTMP. Response to treatment was graded as good (pain reduction of 50-100%), intermediate (25-49%) and poor (0-24%).
Results: All patients showed good uptake of 153Sm-EDTMP by bone metastases. Among the patients with prostate cancer, intermediate or good response to therapy occurred in 80.6% (25 patients) and poor response in 19.4% (6). Among the patients with breast cancer, 85% (17) showed intermediate or good response to therapy while 15% (3) showed poor response. All three patients with lung cancer showed poor response to treatment. The lung hemangioendothelioma and unknown primary lesion patients showed intermediate response to treatment; the osteosarcoma and parathyroid adenocarcinoma patients showed good response to treatment. No significant myelotoxicity occurred.
Discussion: Pain control is important for improving the quality of life of patients with advanced cancers. The mechanism by which pain is relieved with the use of radionuclides is still not yet completely understood, however, the treatment is simple and provides a low risk of mielotoxicity.
Conclusion: Treatment with 153Sm-EDTMP can control the pain secondary to bone metastases effectively in most patients with breast and prostate cancer without significant side effects.
CONTEXTO:: Mais de 50% dos pacientes com câncer de próstata, mama ou pulmão desenvolverão dor óssea secundária a metástases. O tratamento da dor óssea metastática visa minimizar a dor, reduzir o uso de opióides e manter os movimentos.
OBJETIVO:: Avaliar o uso de EDTMP-153Sm para tratamento da dor óssea secundária a metástases refratária a tratamento com opióides.
TIPO DE ESTUDO:: Retrospectivo.
LOCAL:: Divisão de Medicina Nuclear, Universi-dade Estadual de Campinas (Unicamp).
MÉTODOS:: 58 pacientes foram estudados (34 homens), com média de idade de 62 anos. 31 pacientes com neoplasia de próstata, 20 com neoplasia de mama, três pacientes com câncer de pulmão, um com hemangioendotelioma de pulmão, um com adenocarcinoma de paratireóide, um com osteosarcoma e um paciente que apresentava um tumor primário desconhecido. Todos apresentavam múltiplas metástases ósseas à cintilografia óssea com MDP-99mTc e foram tratados com EDTMP-153Sm. A resposta ao tratamento foi graduada em boa (redução da dor em 50 - 100%), intermediária (25-49%) e má (0-24%).
RESULTADOS:: Todos os pacientes apresentavam boa captação de EDTMP-153Sm nas metástases ósseas. Dentre os doentes com câncer de próstata, resposta intermediária ou boa ocorreu em 80.6% (25 pacientes) e má resposta em 19.4% (6). Dentre os pacientes com câncer de mama, 85% (17) apresentaram resposta intermediária ou boa à terapia enquanto 15% (3) apresentaram má resposta. Todos os três pacientes com câncer de pulmão apresentaram resposta pobre ao tratamento. Os doentes com hemangioendotelioma de pulmão e com o tumor primário desconhecido apresentaram resposta intermediária ao tratamento; os pacientes com osteossarcoma e com o adenocarcinoma de paratireóide apresentaram boa resposta. Mielotoxicidade significativa não ocorreu.
DISCUSSÃO:: O controle da dor é importante para melhorar a qualidade de vida do doente com câncer avançado. O mecanismo de alívio da dor com radionuclídeos ainda não foi elucidado, mas o tratamento é de simples administração e baixo risco de mielotoxidade.
CONCLUSÃO:: Tratamento com EDTMP-153Sm pode controlar a dor secundária a metástases ósseas de forma efetiva na maioria dos pacientes com câncer de próstata e câncer de mama sem efeitos colaterais significativos.
Figures
Similar articles
-
[Primary investigation of dose-effect relationship of 153Sm-EDTMP in treating multiple bone metastases].Ai Zheng. 2006 Nov;25(11):1395-8. Ai Zheng. 2006. PMID: 17094908 Chinese.
-
Palliation of pain associated with metastatic bone cancer using samarium-153 lexidronam: a double-blind placebo-controlled clinical trial.J Clin Oncol. 1998 Apr;16(4):1574-81. doi: 10.1200/JCO.1998.16.4.1574. J Clin Oncol. 1998. PMID: 9552068 Clinical Trial.
-
Prospective evaluation of samarium-153-EDTMP radionuclide treatment for bone metastases in patients with hormone-refractory prostate cancer.Urol Int. 2007;78(1):50-7. doi: 10.1159/000096935. Urol Int. 2007. PMID: 17192733
-
Samarium for osteoblastic bone metastases and osteosarcoma.Expert Opin Pharmacother. 2006 Aug;7(11):1475-86. doi: 10.1517/14656566.7.11.1475. Expert Opin Pharmacother. 2006. PMID: 16859431 Review.
-
Samarium lexidronam (153Sm-EDTMP): skeletal radiation for osteoblastic bone metastases and osteosarcoma.Expert Rev Anticancer Ther. 2007 Nov;7(11):1517-27. doi: 10.1586/14737140.7.11.1517. Expert Rev Anticancer Ther. 2007. PMID: 18020921 Review.
Cited by
-
Personalized Radiation Therapy in Cancer Pain Management.Cancers (Basel). 2019 Mar 19;11(3):390. doi: 10.3390/cancers11030390. Cancers (Basel). 2019. PMID: 30893954 Free PMC article. Review.
-
Biocompatibility and Osteogenic Activity of Samarium-Doped Hydroxyapatite-Biomimetic Nanoceramics for Bone Regeneration Applications.Biomimetics (Basel). 2024 May 22;9(6):309. doi: 10.3390/biomimetics9060309. Biomimetics (Basel). 2024. PMID: 38921189 Free PMC article.
-
EANM guidelines for radionuclide therapy of bone metastases with beta-emitting radionuclides.Eur J Nucl Med Mol Imaging. 2018 May;45(5):846-859. doi: 10.1007/s00259-018-3947-x. Epub 2018 Feb 16. Eur J Nucl Med Mol Imaging. 2018. PMID: 29453701 Free PMC article.
-
Beyond Palliation: Therapeutic Applications of 153Samarium-EDTMP.Clin Exp Pharmacol. 2013 Jun;3(3):1000131. doi: 10.4172/2161-1459.1000131. Clin Exp Pharmacol. 2013. PMID: 25664221 Free PMC article.
References
-
- Holmes RA. [153Sm] EDTMP: a potential therapy for bone cancer pain. Semin Nucl Med. 1992;22(1):41–45. - PubMed
-
- Silberstein E. Treatment of the pain of bone metastases. In: Collier BD, Fogelman I, Rosenthal L, editors. Skeletal Nuclear Medicine. St. Louis: Mosby Year Book; 1996. pp. 468–474.
-
- Campa JA, 3rd, Payne R. The management of intractable bone pain: a clinician's perspective. Semin Nucl Medicine. 1992;22(1):3–10. - PubMed
-
- Resche I, Chatal JF, Pecking A, et al. A dose-controlled study of Sm-ethylenediaminetetramethylenephosphonate (EDTMP) in the treatment of patients with painful bone metastases. Eur J Cancer. 1997;33(10):1583–1591. - PubMed
-
- Collins C, Eary JF, Donaldson G, et al. Samarium-153-EDTMP in bone metastases of hormone refractory prostate carcinoma: a phase I/II trial. J Nucl Med. 1993;34(11):1839–1844. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

