The in vitro effect of gefitinib ('Iressa') alone and in combination with cytotoxic chemotherapy on human solid tumours
- PMID: 15560844
- PMCID: PMC535559
- DOI: 10.1186/1471-2407-4-83
The in vitro effect of gefitinib ('Iressa') alone and in combination with cytotoxic chemotherapy on human solid tumours
Abstract
Background: Activation of the epidermal growth factor receptor (EGFR) triggers downstream signaling pathways that regulate many cellular processes involved in tumour survival and growth. Gefitinib ('Iressa') is an orally active tyrosine kinase inhibitor (TKI) targeted to the ATP-binding domain of EGFR (HER1; erbB1).
Methods: In this study we have used a standardised ATP-based tumour chemosensitivity assay (ATP-TCA) to measure the activity of gefitinib alone or in combination with different cytotoxic drugs (cisplatin, gemcitabine, oxaliplatin and treosulfan) against a variety of solid tumours (n = 86), including breast, colorectal, oesophageal and ovarian cancer, carcinoma of unknown primary site, cutaneous and uveal melanoma, non-small cell lung cancer (NSCLC) and sarcoma. The IC50 and IC90 were calculated for each single agent or combination. To allow comparison between samples the IndexSUM was calculated based on the percentage tumour growth inhibition (TGI) at each test drug concentration (TDC). Gefitinib was tested at concentrations ranging from 0.0625-2 microM (TDC = 0.446 microg/ml). This study represents the first use of a TKI in the assay.
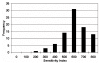
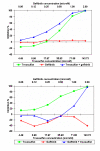
Results: There was heterogeneity in the degree of TGI observed when tumours were tested against single agent gefitinib. 7% (6/86) of tumours exhibited considerable inhibition, but most showed a more modest response resulting in a low TGI. The median IC50 value for single agent gefitinib in all tumours tested was 3.98 microM. Interestingly, gefitinib had both positive and negative effects when used in combination with different cytotoxics. In 59% (45/76) of tumours tested, the addition of gefitinib appeared to potentiate the effect of the cytotoxic agent or combination (of these, 11% (5/45) had a >50% decrease in their IndexSUM). In 38% of tumours (29/76), the TGI was decreased when the combination of gefitinib + cytotoxic was used in comparison to the cytotoxic alone. In the remaining 3% (2/76) there was no change observed.
Conclusion: The in vitro model suggests that gefitinib may have differential effects in response to concomitant cytotoxic chemotherapy with the agents tested during this study. The mechanism involved may relate to the effect of TKIs on growth rate versus their effect on the ability of the cell to survive the stimulus to apoptosis produced by chemotherapy.
Figures



Similar articles
-
Gefitinib and chemotherapy combination studies in five novel human non small cell lung cancer xenografts. Evidence linking EGFR signaling to gefitinib antitumor response.Int J Cancer. 2007 Apr 1;120(7):1579-90. doi: 10.1002/ijc.22364. Int J Cancer. 2007. PMID: 17205515
-
Epidermal Growth Factor Receptor Mutation (EGFR) Testing for Prediction of Response to EGFR-Targeting Tyrosine Kinase Inhibitor (TKI) Drugs in Patients with Advanced Non-Small-Cell Lung Cancer: An Evidence-Based Analysis.Ont Health Technol Assess Ser. 2010;10(24):1-48. Epub 2010 Dec 1. Ont Health Technol Assess Ser. 2010. PMID: 23074402 Free PMC article.
-
Gefitinib in combination with gemcitabine and cisplatin in advanced non-small-cell lung cancer: a phase III trial--INTACT 1.J Clin Oncol. 2004 Mar 1;22(5):777-84. doi: 10.1200/JCO.2004.08.001. J Clin Oncol. 2004. PMID: 14990632 Clinical Trial.
-
Gefitinib (Iressa, ZD1839): a novel targeted approach for the treatment of solid tumors.Bull Cancer. 2004 May 1;91(5):E70-6. Bull Cancer. 2004. PMID: 15582898 Review.
-
New oxaliplatin-based combinations in the treatment of colorectal cancer.Colorectal Dis. 2003 Nov;5 Suppl 3:1-9. doi: 10.1046/j.1463-1318.5.s3.2.x. Colorectal Dis. 2003. PMID: 23573555 Review.
Cited by
-
The Influence of EGFR Inactivation on the Radiation Response in High Grade Glioma.Int J Mol Sci. 2018 Jan 12;19(1):229. doi: 10.3390/ijms19010229. Int J Mol Sci. 2018. PMID: 29329222 Free PMC article.
-
Targeting EGFR for treatment of glioblastoma: molecular basis to overcome resistance.Curr Cancer Drug Targets. 2012 Mar;12(3):197-209. doi: 10.2174/156800912799277557. Curr Cancer Drug Targets. 2012. PMID: 22268382 Free PMC article. Review.
-
Naturally occurring resistance of bone marrow mononuclear and metastatic cancer cells to anticancer agents.Clin Exp Metastasis. 2006;23(5-6):249-58. doi: 10.1007/s10585-006-9034-x. Epub 2006 Nov 3. Clin Exp Metastasis. 2006. PMID: 17086360
-
An EGFR inhibitor enhances the efficacy of SN38, an active metabolite of irinotecan, in SN38-refractory gastric carcinoma cells.Br J Cancer. 2011 Nov 8;105(10):1522-32. doi: 10.1038/bjc.2011.397. Epub 2011 Oct 13. Br J Cancer. 2011. PMID: 21997136 Free PMC article.
-
Recent advances on skin-resident stem/progenitor cell functions in skin regeneration, aging and cancers and novel anti-aging and cancer therapies.J Cell Mol Med. 2010 Jan;14(1-2):116-34. doi: 10.1111/j.1582-4934.2009.00885.x. Epub 2009 Sep 1. J Cell Mol Med. 2010. PMID: 19725922 Free PMC article. Review.
References
-
- Wakeling AE, Guy SP, Woodburn JR, Ashton SE, Curry BJ, Barker AJ, Gibson KH. ZD1839 (Iressa): An Orally Active Inhibitor of Epidermal Growth Factor Signaling with Potential for Cancer Therapy. Cancer Research. 2002;62:5749–5754. - PubMed
-
- Ranson M, Hammond LA, Ferry D, Kris M, Tullo A, Murray PI, Miller V, Averbuch S, Ochs J, Morris C, Feyereislova A, Swaisland H, Rowinsky EK. ZD1839, a selective oral epidermal growth factor receptor-tyrosine kinase inhibitor, is well tolerated and active in patients with solid, malignant tumors: results of a phase I trial. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology. 2002;20:2240–2250. - PubMed
-
- Fukuoka M, Yano S, Giaccone G, Tamura T, Nakagawa K, Douillard JY, Nishiwaki Y, Vansteenkiste J, Kudoh S, Rischin D, Eek R, Horai T, Noda K, Takata I, Smit E, Averbuch S, Macleod A, Feyereislova A, Dong RP, Baselga J. Multi-institutional randomized phase II trial of gefitinib for previously treated patients with advanced non-small-cell lung cancer. Journal of Clinical Oncology. 2003;21:2237–2246. doi: 10.1200/JCO.2003.10.038. - DOI - PubMed
-
- Kris MG, Natale RB, Herbst RS, Lynch TJ, Jr, Prager D, Belani CP, Schiller JH, Kelly K, Spiridonidis H, Sandler A, Albain KS, Cella D, Wolf MK, Averbuch SD, Ochs JJ, Kay AC. Efficacy of gefitinib, an inhibitor of the epidermal growth factor receptor tyrosine kinase, in symptomatic patients with non-small cell lung cancer: a randomized trial. JAMA. 2003;290:2149–2158. doi: 10.1001/jama.290.16.2149. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

