Chalcomycin biosynthesis gene cluster from Streptomyces bikiniensis: novel features of an unusual ketolide produced through expression of the chm polyketide synthase in Streptomyces fradiae
- PMID: 15561847
- PMCID: PMC529187
- DOI: 10.1128/AAC.48.12.4703-4712.2004
Chalcomycin biosynthesis gene cluster from Streptomyces bikiniensis: novel features of an unusual ketolide produced through expression of the chm polyketide synthase in Streptomyces fradiae
Abstract
Chalcomycin, a 16-membered macrolide antibiotic made by the bacterium Streptomyces bikiniensis, contains a 2,3-trans double bond and the neutral sugar D-chalcose in place of the amino sugar mycaminose found in most other 16-membered macrolides. Degenerate polyketide synthase (PKS)-specific primers were used to amplify DNA fragments from S. bikiniensis with very high identity to a unique ketosynthase domain of the tylosin PKS. The resulting amplimers were used to identify two overlapping cosmids encompassing the chm PKS. Sequencing revealed a contiguous segment of >60 kb carrying 25 putative genes for biosynthesis of the polyketide backbone, the two deoxysugars, and enzymes involved in modification of precursors of chalcomycin or resistance to it. The chm PKS lacks the ketoreductase and dehydratase domains in the seventh module expected to produce the 2,3-double bond in chalcomycin. Expression of PKS in the heterologous host Streptomyces fradiae, from which the tyl genes encoding the PKS had been removed, resulted in production of at least one novel compound, characterized as a 3-keto 16-membered macrolactone in equilibrium with its 3-trans enol tautomer and containing the sugar mycaminose at the C-5 position, in agreement with the structure predicted on the basis of the domain organization of the chm PKS. The production of a 3-keto macrolide from the chm PKS indicates that a discrete set of enzymes is responsible for the introduction of the 2,3-trans double bond in chalcomycin. From comparisons of the open reading frames to sequences in databases, a pathway for the synthesis of nucleoside diphosphate-D-chalcose was proposed.
Figures
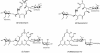

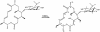


References
-
- Anzai, Y., N. Saito, M. Tanaka, K. Kinoshita, Y. Koyama, and F. Kato. 2003. Organization of the biosynthetic gene cluster for the polyketide macrolide mycinamicin in Micromonospora griseorubida. FEMS Microbiol. Lett. 218:135-141. - PubMed
-
- Asolkar, R. N., R. P. Maskey, E. Helmke, and H. Laatsch. 2002. Chalcomycin B, a new macrolide antibiotic from the marine isolate Streptomyces sp. B7064. J. Antibiot. 55:893-898. - PubMed
-
- Baltz, R. H., and E. T. Seno. 1988. Genetics of Streptomyces fradiae and tylosin biosynthesis. Annu. Rev. Microbiol. 42:547-574. - PubMed
-
- Bierman, M., R. Logan, K. O'Brien, E. T. Seno, R. N. Rao, and B. E. Schoner. 1992. Plasmid cloning vectors for the conjugal transfer of DNA from Escherichia coli to Streptomyces spp. Gene 116:43-49. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

