Antistaphylococcal activity of WCK 771, a tricyclic fluoroquinolone, in animal infection models
- PMID: 15561853
- PMCID: PMC529196
- DOI: 10.1128/AAC.48.12.4754-4761.2004
Antistaphylococcal activity of WCK 771, a tricyclic fluoroquinolone, in animal infection models
Abstract
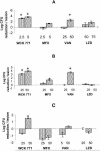
WCK 771, the arginine salt of S-(-)-nadifloxacin, was evaluated in animal models of staphylococcal infection and in vitro. For 302 methicillin-susceptible strains the MIC at which 50% of isolates are inhibited (MIC50) and the MIC90 of WCK 771 were 0.03 and 0.03 microg/ml, respectively, and for 198 methicillin-resistant strains the MIC50 and the MIC90 were 0.5 and 1.0 microg/ml, respectively. All methicillin-susceptible staphylococci were quinolone susceptible, and almost all methicillin-resistant staphylococci were quinolone resistant. WCK 771 was more potent than moxifloxacin, trovafloxacin, levofloxacin, and ciprofloxacin and had potency comparable to that of clinafloxacin. Only WCK 771 and clinafloxacin demonstrated strong potencies against vancomycin-intermediate Staphylococcus aureus strains (MICs = 1 microg/ml). WCK 771 is not a substrate of the NorA pump, as evident from the lack of an effect of reserpine on the MICs and similar protective doses against infections caused by efflux-positive and -negative staphylococci. WCK 771 was effective by both the oral and the subcutaneous routes in mice infected intraperitoneally with quinolone-susceptible methicillin-susceptible S. aureus (MSSA) strains. For infections caused by quinolone-resistant methicillin-resistant S. aureus (MRSA) strains, the activity of WCK 771 administered subcutaneously was superior to those of trovafloxacin and sparfloxacin, with a 50% effective dose range of 27.8 to 46.8 mg/kg of body weight. The activity of WCK 771 was superior to those of moxifloxacin, vancomycin, and linezolid in a mouse cellulitis model of infection caused by one MSSA and two MRSA strains, with effective doses of 2.5 and 5 mg/kg for the MSSA strain and 10-fold higher effective doses for MRSA strains. WCK 771, like vancomycin and linezolid, eradicated MRSA from mouse liver, spleen, kidney, and lung when it was administered subcutaneously at a dose of 50 mg/kg for four doses. These studies have demonstrated the effectiveness of WCK 771, administered orally and parenterally, for the treatment of diverse staphylococcal infections in mice, including those caused by quinolone-resistant strains.
Figures

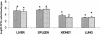
References
-
- Aeschlimann, J. R., L. D. Dresser, G. W. Kaatz, and M. J. Rybak. 1999. Effects of NorA inhibitors on in vitro antibacterial activities and postantibiotic effects of levofloxacin, ciprofloxacin, and norfloxacin in genetically related strains of Staphylococcus aureus. Antimicrob. Agents Chemother. 43:335-340. - PMC - PubMed
-
- Boswell, F. J., J. P. Ashby, J. M. Andrews, and R. Wise. 2002. Effect of protein binding on the in vitro activity and pharmacodynamics of faropenem. J. Antimicrob. Chemother. 50:525-532. - PubMed
-
- Bozdogan, B., D. Esel, C. Whitener, F. A. Browne, and P. C. Appelbaum. 2003. Antibacterial susceptibility of a vancomycin-resistant Staphylococcus aureus strain isolated at the Hershey Medical Center. J. Antimicrob. Chemother. 52:864-868. - PubMed
-
- Centers for Disease Control and Prevention. 1997. Reduced susceptibility of Staphylococcus aureus to vancomycin—Japan, 1996. Morb. Mortal. Wkly. Rep. 46:624-626. - PubMed
-
- Chang, S., D. M. Sievert, J. C. Hageman, M. L. Boulton, F. C. Tenover, F. P. Downes, S. Shah, J. T. Rudrik, G. R. Pupp, W. J. Brown, D. Cardo, and S. K. Fridkin. 2003. Infection with vancomycin-resistant Staphylococcus aureus containing the vanA resistance gene. N. Engl. J. Med. 348:1342-1347. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

