Pharmacokinetics in animals and humans of a first-in-class peptide deformylase inhibitor
- PMID: 15561864
- PMCID: PMC529202
- DOI: 10.1128/AAC.48.12.4835-4842.2004
Pharmacokinetics in animals and humans of a first-in-class peptide deformylase inhibitor
Abstract
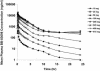
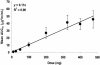
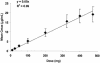
BB-83698, a potent and selective inhibitor of peptide deformylase, was the first compound of this novel antibacterial class to progress to clinical trials. Single- and/or multiple-dose studies with doses ranging from 10 to 50 mg of BB-83698/kg of body weight were done with mice, rats, and dogs. Intravenous pharmacokinetics were characterized by low to moderate clearances and moderate volumes of distribution for all species. In dogs, but not in rodents, central nervous system (CNS) effects were dose limiting for intravenously administered BB-83698 and were suspected to be related to a high maximum concentration of the agent in plasma (Cmax) rather than to total systemic exposure. Controlled infusion studies with dogs demonstrated that CNS effects could be avoided without compromising systemic exposure by reducing the Cmax. A randomized, double-blind, placebo-controlled, five-way-crossover, single-dose-escalation, phase I study to explore the safety, tolerability, and pharmacokinetics of intravenous BB-83698 at doses ranging from 10 to 475 mg was performed with healthy male volunteers. Systemic exposures were generally in linear relationships with administered doses in animals and humans. Pharmacokinetics were consistent, predictable, and exhibited good allometric scaling among all species (r2 >0.98). Moreover, BB-83698 dosing in humans proceeded to a predicted efficacious exposure (the area under the concentration-time curve/MIC ratio, up to 184) without any clinically significant adverse effects.
Figures




References
-
- Boxenbaum, H. 1982. Interspecies scaling, allometry, physiological time, and the ground plan of pharmacokinetics. J. Pharmacokinet. Biopharm. 10:201-227. - PubMed
-
- Clements, J. M., R. P. Beckett, A. Brown, G. Catlin, M. Lobell, S. Palan, W. Thomas, M. Whittaker, S. Wood, S. Salama, P. J. Baker, H. F. Rodgers, V. Barynin, D. W. Rice, and M. G. Hunter. 2001. Antibiotic activity and characterization of BB-3497, a novel peptide deformylase inhibitor. Antimicrob. Agents Chemother. 45:563-570. - PMC - PubMed
-
- Clements, J. M., A. P. Ayscough, K. Keavey, and S. P. East. 2002. Peptide deformylase inhibitors, potential for a new class of broad spectrum antibacterials. Curr. Med. Chem. Anti-Infect. Agents 1:239-249.
-
- Davies, S. J., A. P. Ayscough, R. P. Beckett, J. M. Clements, S. Doel, L. M. Pratt, Z. M. Spavold, S. W. Thomas, and M. Whittaker. 2003. Structure-activity relationships of the peptide deformylase inhibitor BB-3497: modification of the P2′ and P3′ side chains. Bioorg. Med. Chem. Lett. 13:2715-2718. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

