Secreted Bacterial Effectors and Host-Produced Eiger/TNF Drive Death in aSalmonella-Infected Fruit Fly
- PMID: 15562316
- PMCID: PMC532388
- DOI: 10.1371/journal.pbio.0020418
Secreted Bacterial Effectors and Host-Produced Eiger/TNF Drive Death in aSalmonella-Infected Fruit Fly
Abstract
Death by infection is often as much due to the host's reaction as it is to the direct result of microbial action. Here we identify genes in both the host and microbe that are involved in the pathogenesis of infection and disease in Drosophila melanogaster challenged with Salmonella enterica serovartyphimurium (S. typhimurium). We demonstrate that wild-typeS. typhimurium causes a lethal systemic infection when injected into the hemocoel of D. melanogaster. Deletion of the gene encoding the secreted bacterial effect or Salmonella leucine-rich (PslrP)changes an acute and lethal infection to one that is persistent and less deadly. We propose a model in which Salmonella secreted effectors stimulate the fly and thus cause an immune response that is damaging both to the bacteria and, subsequently, to the host. In support of this model, we show that mutations in the fly gene eiger, a TNF homolog, delay the lethality of Salmonella infection. These results suggest that S. typhimurium-infected flies die from a condition that resembles TNF-induced metabolic collapse in vertebrates. This idea provides us with a new model to study shock-like biology in a genetically manipulable host. In addition, it allows us to study the difference in pathways followed by a microbe when producing an acute or persistent infection.
Conflict of interest statement
The authors have declared that no conflicts of interest exist.
Figures
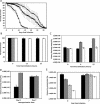


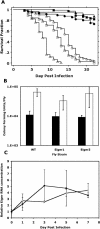
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

