Heterocyclic peptide backbone modifications in an alpha-helical coiled coil
- PMID: 15563148
- PMCID: PMC1868409
- DOI: 10.1021/ja0450408
Heterocyclic peptide backbone modifications in an alpha-helical coiled coil
Abstract
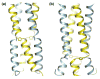
In this paper, we present 1,2,3-triazole epsilon2-amino acids incorporated as a dipeptide surrogate at three positions in the sequence of a known alpha-helical coiled coil. Biophysical characterization indicates that the modified peptides retain much of the helical structure of the parent sequence, and that the thermodynamic stability of the coiled coil depends on the position of the incorporation of the epsilon-residue. Crystal structures obtained for each peptide give insight into the chemical behavior and conformational preferences of the non-natural amino acid and show that the triazole ring can participate in the backbone hydrogen bonding of the alpha-helix as well as template an interhelical crossing between chains in the bundle.
Figures

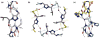
Similar articles
-
Crystal structure of a synthetic triple-stranded alpha-helical bundle.Science. 1993 Feb 26;259(5099):1288-93. doi: 10.1126/science.8446897. Science. 1993. PMID: 8446897
-
Conformational stability of helical peptides containing a thioamide linkage.Org Lett. 2002 Dec 26;4(26):4655-7. doi: 10.1021/ol027056d. Org Lett. 2002. PMID: 12489953
-
A parallel coiled-coil tetramer with offset helices.Biochemistry. 2006 Dec 26;45(51):15224-31. doi: 10.1021/bi061914m. Epub 2006 Nov 29. Biochemistry. 2006. PMID: 17176044
-
Protein design: a hierarchic approach.Science. 1995 Nov 10;270(5238):935-41. doi: 10.1126/science.270.5238.935. Science. 1995. PMID: 7481798 Review.
-
Native-like and structurally characterized designed alpha-helical bundles.Curr Opin Struct Biol. 1995 Aug;5(4):457-63. doi: 10.1016/0959-440x(95)80029-8. Curr Opin Struct Biol. 1995. PMID: 8528761 Review.
Cited by
-
Protein backbone engineering as a strategy to advance foldamers toward the frontier of protein-like tertiary structure.Org Biomol Chem. 2014 Nov 28;12(44):8796-802. doi: 10.1039/c4ob01769b. Org Biomol Chem. 2014. PMID: 25285575 Free PMC article. Review.
-
Synthesis and Biological Properties of EGFR-Targeted Photosensitizer Based on Cationic Porphyrin.Pharmaceutics. 2023 Apr 19;15(4):1284. doi: 10.3390/pharmaceutics15041284. Pharmaceutics. 2023. PMID: 37111769 Free PMC article.
-
Nonpeptidic tetrafluorophenoxymethyl ketone cruzain inhibitors as promising new leads for Chagas disease chemotherapy.J Med Chem. 2010 Feb 25;53(4):1763-73. doi: 10.1021/jm901633v. J Med Chem. 2010. PMID: 20088534 Free PMC article.
-
Overcoming synthetic challenges of oridonin A-ring structural diversification: regio- and stereoselective installation of azides and 1,2,3-triazoles at the C-1, C-2, or C-3 position.Org Lett. 2013 Jul 19;15(14):3718-21. doi: 10.1021/ol4015865. Epub 2013 Jul 8. Org Lett. 2013. PMID: 23834026 Free PMC article.
-
1,2,3-Triazoles as Biomimetics in Peptide Science.Molecules. 2021 May 14;26(10):2937. doi: 10.3390/molecules26102937. Molecules. 2021. PMID: 34069302 Free PMC article. Review.
References
-
-
For reviews on the subject, see: Spatola AF. Chemistry and Biochemistry of Amino Acids, Peptides, and Proteins. Dekker; New York: 1983. pp. 267–357.Ahn JM, Boyle NA, MacDonald MT, Janda KD. Mini-Rev Med Chem. 2002;2:463–473.
-
-
- Chapman E, Thorson JS, Schultz PG. J Am Chem Soc. 1997;119:7151–7152.
- Beligere GS, Dawson PE. J Am Chem Soc. 2000;122:12079–12082.
- Wales TE, Fitzgerald MC. J Am Chem Soc. 2001;123:7709–7710. - PubMed
- Deechongkit S, Nguyen H, Powers ET, Dawson PE, Gruebele M, Kelly JW. Nature. 2004;430:101–105. - PubMed
-
-
For a description of the ε2 nomenclature, see Figure S1.
-
-
- Wang Q, Chan TR, Hilgraf R, Fokin VV, Sharpless KB, Finn MG. J Am Chem Soc. 2003;125:3192–3193. - PubMed
- Link AJ, Tirrell DA. J Am Chem Soc. 2003;125:11164–11165. - PubMed
- Deiters A, Cropp TA, Mukherji M, Chin JW, Anderson JC, Schultz PG. J Am Chem Soc. 2003;125:11782–11783. - PubMed
- Fazio F, Bryan MC, Blixt O, Paulson JC, Wong CH. J Am Chem Soc. 2002;124:14397–14402. - PubMed
- Speers AE, Adam GC, Cravatt BF. J Am Chem Soc. 2003;125:4686–4687. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

