The use of pharmacokinetic and pharmacodynamic data in the assessment of drug safety in early drug development
- PMID: 15563358
- PMCID: PMC1884636
- DOI: 10.1111/j.1365-2125.2004.02194.x
The use of pharmacokinetic and pharmacodynamic data in the assessment of drug safety in early drug development
Abstract
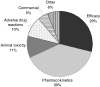
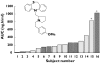
The pharmaceutical industry continues to look for ways to reduce drug candidate attrition throughout the drug discovery and development process. A significant cause of attrition is due to safety issues arising either as a result of animal toxicity testing or in the clinical programme itself. A factor in the assessment of safety during early drug development is the pharmacokinetic profile of the compound. This allows safety data to be considered in the light of systemic drug exposure and therefore permits a quantitative assessment. This is particularly applicable when assessing the risk of a new chemical entity (NCE) in relation to safety parameters such as QT interval prolongation, where free plasma concentrations have been shown to be predictive of this property in relation to potency in preclinical testing. Prior to actual human exposure it is therefore important to be able to predict reliably the pharmacokinetic behaviour of an NCE in order to place such safety findings into a quantitative risk context. The emerging science of pharmacogenetics is likely to further our ability to assess the risk of NCEs to populations and individuals due to genetic variance. The drug metabolizing enzyme CYP2D6 has been recognized as providing the potential to result in widely differing systemic drug exposure in the patient population due to polymorphic expression. Further knowledge is likely to add to our understanding of population differences in exposure and response and aid in the identification of risk factors. One potential strategy for improving the effectiveness of the drug discovery process is to obtain clinical pharmacokinetic data more rapidly in order to assess more accurately the potential for both efficacy and safety of an NCE. Whilst procedures and technologies are available that allow this on the microdose scale, it is important that we recognize potential limitations of these approaches in order that they can be applied beneficially.
Figures


Similar articles
-
[Clinical-pharmacological aspects to accelerate the development process from the preclinical to the clinical phase/2nd communication: promising strategies].Arzneimittelforschung. 2004;54(6):307-13. doi: 10.1055/s-0031-1296976. Arzneimittelforschung. 2004. PMID: 15282874 Review. German.
-
[Clinical-pharmacological aspects to accelerate the development process from the preclinical to the clinical phase/1st communication: The contribution of clinical pharmacology].Arzneimittelforschung. 2004;54(5):251-8. doi: 10.1055/s-0031-1296967. Arzneimittelforschung. 2004. PMID: 15212186 German.
-
A Historical View and Vision into the Future of the Field of Safety Pharmacology.Handb Exp Pharmacol. 2015;229:3-45. doi: 10.1007/978-3-662-46943-9_1. Handb Exp Pharmacol. 2015. PMID: 26091634 Review.
-
Exploratory drug safety: a discovery strategy to reduce attrition in development.J Pharmacol Toxicol Methods. 2009 Jul-Aug;60(1):69-78. doi: 10.1016/j.vascn.2009.04.194. Epub 2009 May 5. J Pharmacol Toxicol Methods. 2009. PMID: 19422924 Review.
-
Safety pharmacology investigations in toxicology studies: an industry survey.J Pharmacol Toxicol Methods. 2013 Jul-Aug;68(1):44-51. doi: 10.1016/j.vascn.2013.05.002. Epub 2013 May 14. J Pharmacol Toxicol Methods. 2013. PMID: 23685201
Cited by
-
Application of the bradford hill criteria to assess the causality of cisapride-induced arrhythmia: a model for assessing causal association in pharmacovigilance.Drug Saf. 2007;30(4):333-46. doi: 10.2165/00002018-200730040-00006. Drug Saf. 2007. PMID: 17408310
-
Acute toxicological evaluation of bone-targeted parathyroid hormone-related peptide (PTHrP) antagonist to inhibit breast cancer metastases to bone.Toxicol Rep. 2025 Jul 12;15:102085. doi: 10.1016/j.toxrep.2025.102085. eCollection 2025 Dec. Toxicol Rep. 2025. PMID: 40697326 Free PMC article.
-
Pharmacokinetic Assessment of Rottlerin from Mallotus philippensis Using a Highly Sensitive Liquid Chromatography-Tandem Mass Spectrometry-Based Bioanalytical Method.ACS Omega. 2021 Nov 24;6(48):32637-32646. doi: 10.1021/acsomega.1c04266. eCollection 2021 Dec 7. ACS Omega. 2021. PMID: 34901612 Free PMC article.
-
Unleashing Nature's potential: a computational approach to discovering novel VEGFR-2 inhibitors from African natural compound using virtual screening, ADMET analysis, molecular dynamics, and MMPBSA calculations.Front Mol Biosci. 2023 Sep 20;10:1227643. doi: 10.3389/fmolb.2023.1227643. eCollection 2023. Front Mol Biosci. 2023. PMID: 37800126 Free PMC article.
-
Real-Time Cellular Thermal Shift Assay to Monitor Target Engagement.ACS Chem Biol. 2022 Sep 16;17(9):2471-2482. doi: 10.1021/acschembio.2c00334. Epub 2022 Sep 1. ACS Chem Biol. 2022. PMID: 36049119 Free PMC article.
References
-
- Grabowski H, Vernon J, DiMasi JA. Returns on research and development for 1990s new drug introductions. Pharmacoeconomics. 2002;20(Suppl. 3):11–29. - PubMed
-
- Di Masi JA. The value of improving the productivity of the drug development process. Pharmacoeconomics. 2002;20(Suppl. 3):1–10. - PubMed
-
- Frantz S, Smith A. New drug approvals for 2002. Nat Rev Drug Discov. 2003;2:95–6. - PubMed
-
- Palmer AM. New horizons in drug metabolism, pharmacokinetics and drug discovery. Drug News Perspect. 2003;16:57–62. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

