Medication reviews in the community: results of a randomized, controlled effectiveness trial
- PMID: 15563363
- PMCID: PMC1884656
- DOI: 10.1111/j.1365-2125.2004.02220.x
Medication reviews in the community: results of a randomized, controlled effectiveness trial
Erratum in
- Br J Clin Pharmacol. 2005 Mar;59(3):376
Abstract
Aims: To examine the effectiveness of a multidisciplinary service model delivering medication review to patients at risk of medication misadventure in the community.
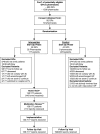
Methods: The study was carried out in three Australian states; Queensland, New South Wales and Western Australia, and conducted as a randomized, controlled effectiveness trial with the general practitioner (GP) as the unit of randomization. In total, 92 GPs, 53 pharmacists and 400 patients enrolled in the study. The multidisciplinary service model consisted of GP education, patient home visits, pharmacist medication reviews, primary healthcare team conferences, GP implementation of action plans in consultation with patients, and follow-up surgery visits for monitoring. Effectiveness was assessed using the four clinical value compass domains of (i) functional status, (ii) clinical outcomes, (iii) satisfaction and (iv) costs. The domains of functional status (assessed by the health-related quality of life measure SF-36 subscales) and clinical outcomes (as assessed by adverse drug events (ADEs), number of GP visits, hospital services and severity of illness) were measured at baseline and endpoint. Satisfaction was measured by success in implementation and by participant satisfaction at endpoint, and costs (as assessed using medication and healthcare service costs, less intervention costs) were measured preintervention and during the trial. In addition, process evaluation was conducted for intervention patients, in which problems and recommendations from the medication reviews were described.
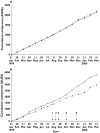
Results: The model was successfully implemented with 92% of intervention GPs suggesting that the model had improved the care of participating patients, a view shared by 94% of pharmacists. In addition, positive trends in clinical outcomes (ADEs and severity of illness) and costs (an ongoing trend towards reduction in healthcare service costs) were evident, although the trial was limited to a 6-month intervention time. No differences between intervention and control groups were identified for the health-related quality of life domain. The cost-effectiveness ratio for the intervention based on cost savings, reduced adverse events and improved health outcomes was small. The most common problems identified in the medication reviews were potential adverse drug reactions, suboptimal monitoring and adherence/lack of concordance issues. In total, 54.4% of recommendations were enacted, and 23.9% were implemented precisely as recommended in the medication review. Follow-up evaluation showed that 70.9% of actions had a positive outcome, 15.7% no effect and 3.7% had a negative outcome.
Conclusions: Most studies emphasize efficacy and the best achievable clinical outcomes rather than whether an intervention will be effective in practice. The current trial showed that three of the four domains in the clinical value compass showed trends of improvement or were indeed improved in the relatively short follow-up period of the trial, suggesting that a service based on this model could achieve similar benefits in practice. A domiciliary medication review programme similar to this model has now been implemented into national Australian practice, where GPs and pharmacists are reimbursed by the Australian government for the provision of these services.
Figures

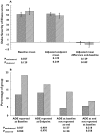
 ), intervention (n = 100) (
), intervention (n = 100) ( ), and (B) patient-reported adverse drug events. Control (n = 180) (
), and (B) patient-reported adverse drug events. Control (n = 180) ( ), intervention (n = 94) (
), intervention (n = 94) ( )
)
References
-
- Stuck AE, Beers MH, Steiner A, Aronow HU, Rubenstein LZ, Beck JC. Inappropriate medication use in community-residing older persons. Arch Intern Med. 1994;154:2195–200. - PubMed
-
- Salzman C. Medication compliance in the elderly. J Clin Psychiatry. 1995;56(Suppl. 1):18–22. - PubMed
-
- Dartnell JG, Anderson RP, Chohan V, et al. Hospitalisation for adverse events related to drug therapy: incidence, avoidability and costs. Med J Aust. 1996;164:659–62. - PubMed
-
- Hallas J, Harvald B, Gram LF, et al. Drug related hospital admissions: the role of definitions and intensity of data collection, and the possibility of prevention. J Intern Med. 1990;228:83–90. - PubMed
-
- Nelson KM, Talbert RL. Drug-related hospital admissions. Pharmacotherapy. 1996;16:701–7. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

