Progression from frontal-parietal to mesial-temporal epilepsy after fluid percussion injury in the rat
- PMID: 15563512
- PMCID: PMC2696356
- DOI: 10.1093/brain/awh337
Progression from frontal-parietal to mesial-temporal epilepsy after fluid percussion injury in the rat
Abstract
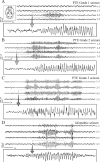
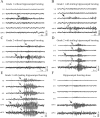
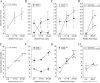
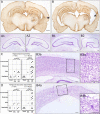
We recently described an in vivo model of post-traumatic epilepsy (PTE) in the rat where chronic spontaneous recurrent seizures appear following a single episode of fluid percussion injury (FPI). PTE, studied during the first 2 months post-injury, was focal and seizures originated predominantly from the frontal-parietal neocortex at or around the injury site. However, rarer bilateral seizures originating from a different and undefined focus were also observed. To shed light on the Posttraumatic Epileptogenic mechanisms and on the generation of bilateral seizures, we studied rats up to 7 months post-injury. In vivo paired epidural and depth-electrode recordings indicated that the anterior hippocampus evolves into an epileptic focus which initiates bilateral seizures. The rate of frontal-parietal seizures remained constant over time after 2 weeks post-injury, while the rate of hippocampal seizures greatly increased over time, suggesting that different mechanisms mediate neocortical and hippocampal post-traumatic epileptogenesis. Because of different temporal evolution of these foci, the epileptic syndrome was characterized by predominant frontal-parietal seizures early after injury, but by predominant mesio-temporal seizures at later time points. Pathological analysis demonstrated progressive hippocampal and temporal cortex pathology that paralleled the increase in frequency and duration of bilateral seizures. These results demonstrate that FPI-induced frontal-parietal epilepsy (FPE) progresses to mesial-temporal lobe epilepsy (MTLE) with dual pathology. These observations establish numerous similarities between FPI-induced and human PTE and further validate it as a clinically relevant model of PTE.
Figures

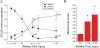
Similar articles
-
Impact of injury location and severity on posttraumatic epilepsy in the rat: role of frontal neocortex.Cereb Cortex. 2011 Jul;21(7):1574-92. doi: 10.1093/cercor/bhq218. Epub 2010 Nov 26. Cereb Cortex. 2011. PMID: 21112931 Free PMC article.
-
Progressive, Seizure-Like, Spike-Wave Discharges Are Common in Both Injured and Uninjured Sprague-Dawley Rats: Implications for the Fluid Percussion Injury Model of Post-Traumatic Epilepsy.J Neurosci. 2015 Jun 17;35(24):9194-204. doi: 10.1523/JNEUROSCI.0919-15.2015. J Neurosci. 2015. PMID: 26085641 Free PMC article.
-
Post-traumatic epilepsy following fluid percussion injury in the rat.Brain. 2004 Feb;127(Pt 2):304-14. doi: 10.1093/brain/awh038. Epub 2003 Nov 7. Brain. 2004. PMID: 14607786 Free PMC article.
-
From traumatic brain injury to posttraumatic epilepsy: what animal models tell us about the process and treatment options.Epilepsia. 2009 Feb;50 Suppl 2:21-9. doi: 10.1111/j.1528-1167.2008.02007.x. Epilepsia. 2009. PMID: 19187291 Review.
-
MTLE with hippocampal sclerosis in adult as a syndrome.Rev Neurol (Paris). 2015 Mar;171(3):259-66. doi: 10.1016/j.neurol.2015.02.004. Epub 2015 Feb 26. Rev Neurol (Paris). 2015. PMID: 25727907 Review.
Cited by
-
A Translational Study on Acute Traumatic Brain Injury: High Incidence of Epileptiform Activity on Human and Rat Electrocorticograms and Histological Correlates in Rats.Brain Sci. 2020 Aug 19;10(9):570. doi: 10.3390/brainsci10090570. Brain Sci. 2020. PMID: 32825101 Free PMC article.
-
Hippocampal cell loss in posttraumatic human epilepsy.Epilepsy Curr. 2007 Nov-Dec;7(6):156-8. doi: 10.1111/j.1535-7511.2007.00177.x. Epilepsy Curr. 2007. PMID: 18049724 Free PMC article. No abstract available.
-
Spike and wave discharges and fast ripples during posttraumatic epileptogenesis.Epilepsia. 2021 Aug;62(8):1842-1851. doi: 10.1111/epi.16958. Epub 2021 Jun 21. Epilepsia. 2021. PMID: 34155626 Free PMC article.
-
Chronic dysfunction of astrocytic inwardly rectifying K+ channels specific to the neocortical epileptic focus after fluid percussion injury in the rat.J Neurophysiol. 2010 Dec;104(6):3345-60. doi: 10.1152/jn.00398.2010. Epub 2010 Sep 22. J Neurophysiol. 2010. PMID: 20861444 Free PMC article.
-
Neural circuit mechanisms of post-traumatic epilepsy.Front Cell Neurosci. 2013 Jun 18;7:89. doi: 10.3389/fncel.2013.00089. eCollection 2013. Front Cell Neurosci. 2013. PMID: 23785313 Free PMC article.
References
-
- Annegers JF, Rocca WA, Hauser WA. Causes of epilepsy: contributions of the Rochester epidemiology project. Mayo Clin Proc. 1996;71:570–575. - PubMed
-
- Annegers JF, Hauser WA, Coan SP, et al. A population-based study of seizures after traumatic brain injuries. N Engl J Med. 1998;338:20–24. - PubMed
-
- Arruda F, Cendes F, Andermann F, Dubeau F, Villemure JG, Jones-Gotman M, Poulin N, Arnold DL, Olivier A. Mesial atrophy and outcome after amygdalohippocampectomy or temporal lobe removal. Ann Neurol. 1996;40(3):446–50. - PubMed
-
- Benbadis SR, Heriaud L, Tatum WO, Vale FL. Epilepsy surgery, delays and referral patterns-are all your epilepsy patients controlled? Seizure. 2003;12(3):167–70. - PubMed
-
- Bertram EH, Zhang DX. Thalamic excitation of hippocampal CA1 neurons: a comparison with the effects of CA3 stimulation. Neuroscience. 1999;92(1):15–26. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

