Critical roles for collagenase-3 (Mmp13) in development of growth plate cartilage and in endochondral ossification
- PMID: 15563592
- PMCID: PMC535367
- DOI: 10.1073/pnas.0407788101
Critical roles for collagenase-3 (Mmp13) in development of growth plate cartilage and in endochondral ossification
Abstract
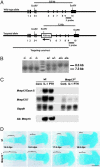
Collagenase-3 (MMP13), a member of the matrix metalloproteinase (MMP) family of neutral endopeptidases, is expressed in the skeleton during embryonic development and is highly overexpressed in human carcinomas and in chondrocytes and synovial cells in rheumatoid arthritis and osteoarthritis. To determine the functional roles of Mmp13, we generated Mmp13-null mice that showed profound defects in growth plate cartilage with markedly increased hypertrophic domains as well as delay in endochondral ossification and formation and vascularization of primary ossification centers. Absence of Mmp13 resulted in significant interstitial collagen accumulation due, in part, to the lack of appropriate collagenase-mediated cleavage that normally occurs in growth plates and primary ossification centers. Cartilaginous growth plate abnormalities persisted in adult mice and phenocopied defects observed in human hereditary chondrodysplasias. Our findings demonstrate a unique role of Mmp13 in skeletal development.
Figures




Similar articles
-
Altered endochondral bone development in matrix metalloproteinase 13-deficient mice.Development. 2004 Dec;131(23):5883-95. doi: 10.1242/dev.01461. Development. 2004. PMID: 15539485 Free PMC article.
-
Developmental regulation of Wnt/beta-catenin signals is required for growth plate assembly, cartilage integrity, and endochondral ossification.J Biol Chem. 2005 May 13;280(19):19185-95. doi: 10.1074/jbc.M414275200. Epub 2005 Mar 10. J Biol Chem. 2005. PMID: 15760903
-
MMP-9/gelatinase B is a key regulator of growth plate angiogenesis and apoptosis of hypertrophic chondrocytes.Cell. 1998 May 1;93(3):411-22. doi: 10.1016/s0092-8674(00)81169-1. Cell. 1998. PMID: 9590175 Free PMC article.
-
Matrix metalloproteinases: role in skeletal development and growth plate disorders.Front Biosci. 2006 May 1;11:1702-15. doi: 10.2741/1916. Front Biosci. 2006. PMID: 16368549 Review.
-
The skeleton: a multi-functional complex organ: the growth plate chondrocyte and endochondral ossification.J Endocrinol. 2011 Nov;211(2):109-21. doi: 10.1530/JOE-11-0048. Epub 2011 Jun 3. J Endocrinol. 2011. PMID: 21642379 Review.
Cited by
-
Histological and molecular characterization of the growing nasal septum in mice.J Anat. 2021 Mar;238(3):751-764. doi: 10.1111/joa.13332. Epub 2020 Oct 12. J Anat. 2021. PMID: 33043993 Free PMC article.
-
Stage-specific secretion of HMGB1 in cartilage regulates endochondral ossification.Mol Cell Biol. 2007 Aug;27(16):5650-63. doi: 10.1128/MCB.00130-07. Epub 2007 Jun 4. Mol Cell Biol. 2007. PMID: 17548469 Free PMC article.
-
MMP13 mutation causes spondyloepimetaphyseal dysplasia, Missouri type (SEMD(MO).J Clin Invest. 2005 Oct;115(10):2832-42. doi: 10.1172/JCI22900. J Clin Invest. 2005. PMID: 16167086 Free PMC article.
-
Egr1 deficiency disrupts dynamic equilibrium of chondrocyte extracellular matrix through PPARγ/RUNX2 signaling pathways.Am J Transl Res. 2018 Jun 15;10(6):1620-1632. eCollection 2018. Am J Transl Res. 2018. PMID: 30018705 Free PMC article.
-
Tissue inhibitor of metalloproteinase-3 (TIMP-3) regulates hematopoiesis and bone formation in vivo.PLoS One. 2010 Sep 30;5(9):e13086. doi: 10.1371/journal.pone.0013086. PLoS One. 2010. PMID: 20941363 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

