Fibronectin matrix turnover occurs through a caveolin-1-dependent process
- PMID: 15563605
- PMCID: PMC545909
- DOI: 10.1091/mbc.e04-08-0672
Fibronectin matrix turnover occurs through a caveolin-1-dependent process
Abstract
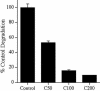
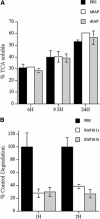
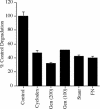
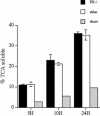
Extracellular matrix remodeling occurs during development, tissue repair, and in a number of pathologies, including fibrotic disorders, hypertension, and atherosclerosis. Extracellular matrix remodeling involves the complex interplay between extracellular matrix synthesis, deposition, and degradation. Factors that control these processes are likely to play key roles in regulating physiological and pathological extracellular matrix remodeling. Our data show that fibronectin polymerization into the extracellular matrix regulates the deposition and stability of other extracellular matrix proteins, including collagen I and thrombospondin-1 (Sottile and Hocking, 2002. Mol. Biol. Cell 13, 3546). In the absence of continual fibronectin polymerization, there is a loss of fibronectin matrix fibrils, and increased levels of fibronectin degradation. Fibronectin degradation occurs intracellularly after endocytosis and can be inhibited by chloroquine, an inhibitor of lysosomal degradation, and by caveolae-disrupting agents. Down-regulation of caveolin-1 by RNAi inhibits loss of fibronectin matrix fibrils, fibronectin internalization, and fibronectin degradation; these processes can be restored by reexpression of caveolin-1. These data show that fibronectin matrix turnover occurs through a caveolin-1-dependent process. Caveolin-1 regulation of fibronectin matrix turnover is a novel mechanism regulating extracellular matrix remodeling.
Figures









Similar articles
-
Fibronectin polymerization regulates the composition and stability of extracellular matrix fibrils and cell-matrix adhesions.Mol Biol Cell. 2002 Oct;13(10):3546-59. doi: 10.1091/mbc.e02-01-0048. Mol Biol Cell. 2002. PMID: 12388756 Free PMC article.
-
Collagen I matrix turnover is regulated by fibronectin polymerization.Am J Physiol Cell Physiol. 2010 May;298(5):C1265-75. doi: 10.1152/ajpcell.00341.2009. Epub 2010 Jan 27. Am J Physiol Cell Physiol. 2010. PMID: 20107040 Free PMC article.
-
Caveolin-1-dependent beta1 integrin endocytosis is a critical regulator of fibronectin turnover.J Cell Sci. 2008 Jul 15;121(Pt 14):2360-71. doi: 10.1242/jcs.014977. Epub 2008 Jun 24. J Cell Sci. 2008. PMID: 18577581 Free PMC article.
-
Basic components of connective tissues and extracellular matrix: elastin, fibrillin, fibulins, fibrinogen, fibronectin, laminin, tenascins and thrombospondins.Adv Exp Med Biol. 2014;802:31-47. doi: 10.1007/978-94-007-7893-1_3. Adv Exp Med Biol. 2014. PMID: 24443019 Review.
-
Fibronectin matrix deposition and cell contractility: implications for airway remodeling in asthma.Chest. 2002 Dec;122(6 Suppl):275S-278S. doi: 10.1378/chest.122.6_suppl.275s. Chest. 2002. PMID: 12475798 Review.
Cited by
-
Matrix fibronectin binds gammaretrovirus and assists in entry: new light on viral infections.J Virol. 2007 Aug;81(15):8247-57. doi: 10.1128/JVI.00312-07. Epub 2007 May 23. J Virol. 2007. PMID: 17522212 Free PMC article.
-
Polypeptide N-acetylgalactosaminyltransferase 6 disrupts mammary acinar morphogenesis through O-glycosylation of fibronectin.Neoplasia. 2011 Apr;13(4):320-6. doi: 10.1593/neo.101440. Neoplasia. 2011. PMID: 21472136 Free PMC article.
-
Different contributions of clathrin- and caveolae-mediated endocytosis of vascular endothelial cadherin to lipopolysaccharide-induced vascular hyperpermeability.PLoS One. 2014 Sep 2;9(9):e106328. doi: 10.1371/journal.pone.0106328. eCollection 2014. PLoS One. 2014. PMID: 25180771 Free PMC article.
-
Cellular uptake of nanoparticles: journey inside the cell.Chem Soc Rev. 2017 Jul 17;46(14):4218-4244. doi: 10.1039/c6cs00636a. Chem Soc Rev. 2017. PMID: 28585944 Free PMC article. Review.
-
Structural and Functional Characterization of Fibronectin in Extracellular Vesicles From Hepatocytes.Front Cell Dev Biol. 2021 Mar 18;9:640667. doi: 10.3389/fcell.2021.640667. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 33816490 Free PMC article.
References
-
- Ali, I. U., and Hynes, R. O. (1978). Role of disulfide bonds in the attachment and function of large, external, transformation-sensitive glycoprotein at the cell surface. Biochem. Biophys. Acta 510, 140-150. - PubMed
-
- Allen-Hoffmann, L., and Mosher, D. F. (1987). Matrix assembly sites for exogenous fibronectin are decreased on human fibroblasts after treatment with agents which increase intracellular cAMP. J. Biol. Chem. 262, 14361-14365. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

