Quantitative imaging of single live cells reveals spatiotemporal dynamics of multistep signaling events of chemoattractant gradient sensing in Dictyostelium
- PMID: 15563608
- PMCID: PMC545903
- DOI: 10.1091/mbc.e04-07-0544
Quantitative imaging of single live cells reveals spatiotemporal dynamics of multistep signaling events of chemoattractant gradient sensing in Dictyostelium
Abstract
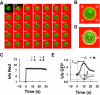
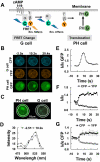
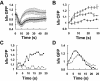
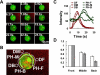
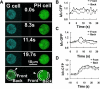
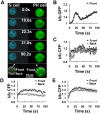
Activation of G-protein-coupled chemoattractant receptors triggers dissociation of Galpha and Gbetagamma subunits. These subunits induce intracellular responses that can be highly polarized when a cell experiences a gradient of chemoattractant. Exactly how a cell achieves this amplified signal polarization is still not well understood. Here, we quantitatively measure temporal and spatial changes of receptor occupancy, G-protein activation by FRET imaging, and PIP3 levels by monitoring the dynamics of PH(Crac)-GFP translocation in single living cells in response to different chemoattractant fields. Our results provided the first direct evidence that G-proteins are activated to different extents on the cell surface in response to asymmetrical stimulations. A stronger, uniformly applied stimulation triggers not only a stronger G-protein activation but also a faster adaptation of downstream responses. When naive cells (which have not experienced chemoattractant) were abruptly exposed to stable cAMP gradients, G-proteins were persistently activated throughout the entire cell surface, whereas the response of PH(Crac)-GFP translocation surprisingly consisted of two phases, an initial transient and asymmetrical translocation around the cell membrane, followed by a second phase producing a highly polarized distribution of PH(Crac)-GFP. We propose a revised model of gradient sensing, suggesting an important role for locally controlled components that inhibit PI3Kinase activity.
Figures



Similar articles
-
Locally controlled inhibitory mechanisms are involved in eukaryotic GPCR-mediated chemosensing.J Cell Biol. 2007 Jul 2;178(1):141-53. doi: 10.1083/jcb.200611096. J Cell Biol. 2007. PMID: 17606871 Free PMC article.
-
Using quantitative fluorescence microscopy and FRET imaging to measure spatiotemporal signaling events in single living cells.Methods Mol Biol. 2006;346:281-96. doi: 10.1385/1-59745-144-4:281. Methods Mol Biol. 2006. PMID: 16957297
-
Distinct roles of PI(3,4,5)P3 during chemoattractant signaling in Dictyostelium: a quantitative in vivo analysis by inhibition of PI3-kinase.Mol Biol Cell. 2006 Apr;17(4):1503-13. doi: 10.1091/mbc.e05-09-0825. Epub 2006 Jan 18. Mol Biol Cell. 2006. PMID: 16421252 Free PMC article.
-
Chemoattractant signaling in dictyostelium discoideum.Annu Rev Cell Dev Biol. 2004;20:223-53. doi: 10.1146/annurev.cellbio.20.011303.132633. Annu Rev Cell Dev Biol. 2004. PMID: 15473840 Review.
-
Gradient sensing during chemotaxis.Curr Opin Cell Biol. 2013 Oct;25(5):532-7. doi: 10.1016/j.ceb.2013.06.007. Epub 2013 Jul 20. Curr Opin Cell Biol. 2013. PMID: 23880435 Review.
Cited by
-
A meta-analysis indicates that the regulation of cell motility is a non-intrinsic function of chemoattractant receptors that is governed independently of directional sensing.Front Immunol. 2022 Oct 20;13:1001086. doi: 10.3389/fimmu.2022.1001086. eCollection 2022. Front Immunol. 2022. PMID: 36341452 Free PMC article.
-
3'-phosphoinositides regulate the coordination of speed and accuracy during chemotaxis.Biophys J. 2008 Oct;95(8):4057-67. doi: 10.1529/biophysj.108.130179. Epub 2008 Aug 1. Biophys J. 2008. PMID: 18676656 Free PMC article.
-
Arrestins function in cAR1 GPCR-mediated signaling and cAR1 internalization in the development of Dictyostelium discoideum.Mol Biol Cell. 2014 Oct 15;25(20):3210-21. doi: 10.1091/mbc.E14-03-0834. Epub 2014 Aug 20. Mol Biol Cell. 2014. PMID: 25143405 Free PMC article.
-
The cAMP-induced G protein subunits dissociation monitored in live Dictyostelium cells by BRET reveals two activation rates, a positive effect of caffeine and potential role of microtubules.Cell Signal. 2018 Aug;48:25-37. doi: 10.1016/j.cellsig.2018.04.005. Epub 2018 Apr 24. Cell Signal. 2018. PMID: 29698704 Free PMC article.
-
Quantitative imaging of Rac1 activity in Dictyostelium cells with a fluorescently labelled GTPase-binding domain from DPAKa kinase.Histochem Cell Biol. 2016 Sep;146(3):267-79. doi: 10.1007/s00418-016-1440-9. Epub 2016 Apr 28. Histochem Cell Biol. 2016. PMID: 27126594
References
-
- Adams, S. R., Harootunian, A. T., Buechler, Y. J., Taylor, S. S., and Tsien, R. Y. (1991). Fluorescence ratio imaging of cyclic AMP in single cells. Nature 349, 694-697. - PubMed
-
- Chung, C. Y., Funamoto, S., and Firtel, R. A. (2001). Signaling pathways controlling cell polarity and chemotaxis. Trends Biochem. Sci. 26, 557-566. - PubMed
-
- Comer, F. I., and Parent, C. A. (2002). PI 3-kinases and PTEN: how opposites chemoattract. Cell 109, 541-544. - PubMed
-
- Devreotes, P., and Janetopoulos, C. (2003). Eukaryotic chemotaxis: distinctions between directional sensing and polarization. J. Biol. Chem. 278, 20445-20448. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

