A sorting nexin PpAtg24 regulates vacuolar membrane dynamics during pexophagy via binding to phosphatidylinositol-3-phosphate
- PMID: 15563611
- PMCID: PMC545876
- DOI: 10.1091/mbc.e04-09-0842
A sorting nexin PpAtg24 regulates vacuolar membrane dynamics during pexophagy via binding to phosphatidylinositol-3-phosphate
Abstract
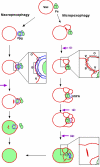
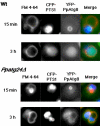
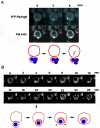
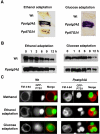
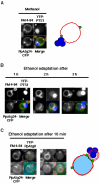
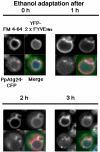
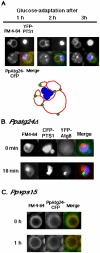
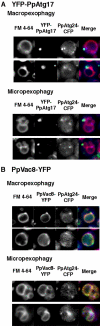
Diverse cellular processes such as autophagic protein degradation require phosphoinositide signaling in eukaryotic cells. In the methylotrophic yeast Pichia pastoris, peroxisomes can be selectively degraded via two types of pexophagic pathways, macropexophagy and micropexophagy. Both involve membrane fusion events at the vacuolar surface that are characterized by internalization of the boundary domain of the fusion complex, indicating that fusion occurs at the vertex. Here, we show that PpAtg24, a molecule with a phosphatidylinositol 3-phosphate-binding module (PX domain) that is indispensable for pexophagy, functions in membrane fusion at the vacuolar surface. CFP-tagged PpAtg24 localized to the vertex and boundary region of the pexophagosome-vacuole fusion complex during macropexophagy. Depletion of PpAtg24 resulted in the blockage of macropexophagy after pexophagosome formation and before the fusion stage. These and other results suggest that PpAtg24 is involved in the spatiotemporal regulation of membrane fusion at the vacuolar surface during pexophagy via binding to phosphatidylinositol 3-phosphate, rather than the previously suggested function in formation of the pexophagosome.
Figures


Similar articles
-
Role of Vac8 in formation of the vacuolar sequestering membrane during micropexophagy.Autophagy. 2006 Oct-Dec;2(4):272-9. doi: 10.4161/auto.3135. Epub 2006 Oct 30. Autophagy. 2006. PMID: 16874085
-
PpAtg30 tags peroxisomes for turnover by selective autophagy.Dev Cell. 2008 Mar;14(3):365-76. doi: 10.1016/j.devcel.2007.12.011. Dev Cell. 2008. PMID: 18331717 Free PMC article.
-
Early and late molecular events of glucose-induced pexophagy in Pichia pastoris require Vac8.Autophagy. 2006 Oct-Dec;2(4):280-8. doi: 10.4161/auto.3164. Epub 2006 Oct 10. Autophagy. 2006. PMID: 16921262
-
Pexophagy: autophagic degradation of peroxisomes.Biochim Biophys Acta. 2006 Dec;1763(12):1767-75. doi: 10.1016/j.bbamcr.2006.08.023. Epub 2006 Aug 24. Biochim Biophys Acta. 2006. PMID: 17005271 Review.
-
Pexophagy in yeasts.Biochim Biophys Acta. 2016 May;1863(5):992-8. doi: 10.1016/j.bbamcr.2015.09.023. Epub 2015 Sep 26. Biochim Biophys Acta. 2016. PMID: 26409485 Review.
Cited by
-
PI4P-signaling pathway for the synthesis of a nascent membrane structure in selective autophagy.J Cell Biol. 2006 Jun 5;173(5):709-17. doi: 10.1083/jcb.200512142. J Cell Biol. 2006. PMID: 16754956 Free PMC article.
-
Active Interaction Mapping Reveals the Hierarchical Organization of Autophagy.Mol Cell. 2017 Feb 16;65(4):761-774.e5. doi: 10.1016/j.molcel.2016.12.024. Epub 2017 Jan 26. Mol Cell. 2017. PMID: 28132844 Free PMC article.
-
Nitrogen Starvation and Stationary Phase Lipophagy Have Distinct Molecular Mechanisms.Int J Mol Sci. 2020 Nov 29;21(23):9094. doi: 10.3390/ijms21239094. Int J Mol Sci. 2020. PMID: 33260464 Free PMC article.
-
Sorting Nexins in Protein Homeostasis.Cells. 2020 Dec 24;10(1):17. doi: 10.3390/cells10010017. Cells. 2020. PMID: 33374212 Free PMC article. Review.
-
A novel fluorescence-activated cell sorting (FACS)-based screening identified ATG14, the gene required for pexophagy in the methylotrophic yeast.FEMS Yeast Res. 2024 Jan 9;24:foae022. doi: 10.1093/femsyr/foae022. FEMS Yeast Res. 2024. PMID: 39025789 Free PMC article.
References
-
- Ago, T., Takeya, R., Hiroaki, H., Kuribayashi, F., Ito, T., Kohda, D., and Sumimoto, H. (2001). The PX domain as a novel phosphoinositide-binding module. Biochem. Biophys. Res. Commun. 287, 733-738. - PubMed
-
- Ausubel, F. M., Brent, R., Kingston, R. E., Moore, D. D., Seidman, J. G., Smith, J. A., and Struhl, K. (eds.) (1987). Current Protocols in Molecular Biology, New York: Greene Publishing Associates and Wiley-Interscience.
-
- Cheever, M. L., Sato, T. K., de Beer, T., Kutateladze, T. G., Emr, S. D., and Overduin, M. (2001). Phox domain interaction with PtdIns(3)P targets the Vam7 t-SNARE to vacuole membranes. Nat. Cell Biol. 3, 613-618. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

