Nodular osteochondrogenic activity in soft tissue surrounding osteoma in neurogenic para osteo-arthropathy: morphological and immunohistochemical study
- PMID: 15563732
- PMCID: PMC543471
- DOI: 10.1186/1471-2474-5-46
Nodular osteochondrogenic activity in soft tissue surrounding osteoma in neurogenic para osteo-arthropathy: morphological and immunohistochemical study
Abstract
Background: Neurogenic Para-Osteo-Arthropathy (NPOA) occurs as a consequence of central nervous system injuries or some systemic conditions. They are characterized by bone formation around the main joints.
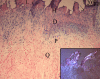
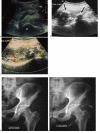
Methods: In order to define some biological features of NPOAs, histological and immunohistological studies of the soft tissue surrounding osteoma and Ultrasound examination (US) of NPOA before the appearance of abnormal ossification on plain radiographs were performed.
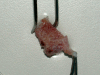
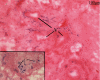
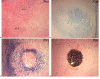
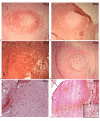
Results: We have observed a great number of ossifying areas scattered in soft tissues. US examination have also shown scattered ossifying areas at the early stage of ossification. A high osteogenic activity was detected in these tissues and all the stages of the endochondral process were observed. Mesenchymal cells undergo chondrocytic differentiation to further terminal maturation with hypertrophy, which sustains mineralization followed by endochondral ossification process.
Conclusion: We suggest that periosteoma soft tissue reflect early stage of osteoma formation and could be a model to study the mechanism of osteoma formation and we propose a mechanism of the NPOA formation in which sympathetic dystony and altered mechanical loading induce changes which could be responsible for the cascade of cellular events leading to cartilage and bone formation.
Figures



Similar articles
-
Cellular origin of endochondral ossification from grafted periosteum.Anat Rec. 2001 Dec 1;264(4):348-57. doi: 10.1002/ar.10024. Anat Rec. 2001. PMID: 11745090
-
Panniculitis ossificans traumatica of the lower leg.Am J Dermatopathol. 2011 Dec;33(8):858-60. doi: 10.1097/DAD.0b013e31820d770b. Am J Dermatopathol. 2011. PMID: 22123193
-
Parosteal osteoma of the iliac bone.Skeletal Radiol. 1998 Nov;27(11):650. doi: 10.1007/s002560050453. Skeletal Radiol. 1998. PMID: 9867185 No abstract available.
-
[Ossification and cutaneous osteoma].Ann Dermatol Venereol. 1994;121(12):918-30. Ann Dermatol Venereol. 1994. PMID: 7632012 Review. French. No abstract available.
-
Juvenile active ossifying fibroma. Its nature, dynamics and origin.Acta Otolaryngol Suppl. 1991;488:1-40. Acta Otolaryngol Suppl. 1991. PMID: 1843064 Review. No abstract available.
Cited by
-
Macrophage-derived oncostatin M contributes to human and mouse neurogenic heterotopic ossifications.JCI Insight. 2017 Nov 2;2(21):e96034. doi: 10.1172/jci.insight.96034. JCI Insight. 2017. PMID: 29093266 Free PMC article.
References
-
- Chantraine A, Nusgens B, Lapiere CM. Biochemical analysis of heterotopic ossification in spinal cord injury patients. Paraplegia. 1995;33:398–401. - PubMed
-
- Dejerine A, Cellier A. Paraosteoarthropathies of paraplegic patients by spinal cord lesion. Clinical and roentgenographic study. Clin Orthop. 1991;263:3–12. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

