Volume sensitivity of the bestrophin family of chloride channels
- PMID: 15564283
- PMCID: PMC1665509
- DOI: 10.1113/jphysiol.2004.075622
Volume sensitivity of the bestrophin family of chloride channels
Abstract
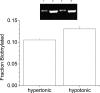
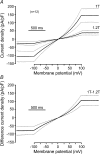
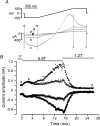
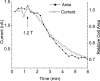
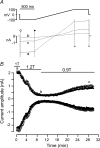
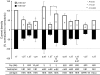
Bestrophins are a newly identified family of Cl(-) channels. Mutations in the founding member of the family, human bestrophin-1 (hBest1), are responsible for a form of early onset macular degeneration called Best vitelliform macular dystrophy. The link between dysfunction of hBest1 and macular degeneration remains unknown. Because retinal pigmented epithelium (RPE) cells may be subjected to varying osmotic pressure due to light-dependent changes in the ionic composition of the subretinal space and because RPE cells may undergo large volume changes during phagocytosis of shed photoreceptor discs, we investigated whether bestrophin currents were affected by cell volume. When hBest1 and mBest2 were overexpressed in HEK 293, HeLa, and ARPE-19 cells, a new Ca(2+)-activated Cl(-) current appeared. This current was very sensitive to cell volume. A 20% increase in extracellular osmolarity caused cell shrinkage and a approximately 70-80% reduction in bestrophin current. Decreases in extracellular osmolarity increased the bestrophin currents slightly, but this was difficult to quantify due to simultaneous activation of endogenous volume-regulated anion channel (VRAC) current. To determine whether a similar current was present in mouse RPE cells, the effect of hyperosmotic solutions on isolated mouse RPE cells was examined. Mouse RPE cells exhibited an endogenous Cl(-) current that resembled the expressed hBest1 in that it was decreased by hypertonic solution. We conclude that bestrophins are volume sensitive and that they could play a novel role in cell volume regulation of RPE cells.
Figures





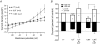
References
-
- Adorante JS. Regulatory volume decrease in frog retinal pigment epithelium. Am J Physiol Cell Physiol. 1995;268:C89–C100. - PubMed
-
- Altamirano J, Brodwick MS, Alvarez-Leefmans FJ. Regulatory volume decrease and intracellular Ca2+ in murine neuroblastoma cells studied with fluorescent probes. J Gen Physiol. 1998;112:145–160. 10.1085/jgp.112.2.145. - DOI - PMC - PubMed
-
- Bakall B, Marmorstein LY, Hoppe G, Peachey NS, Wadelius C, Marmorstein AD. Expression and localization of bestrophin during normal mouse development. Invest Ophthalmol Vis Sci. 2003;44:3622–3628. - PubMed
-
- Besharse JC. The daily light-dark cycle and rhythmic metabolism in the photoreceptor pigment epithelial complex. Prog Retinal Res. 1982;1:81–124.
-
- Caldwell GM, Kakuk LE, Griesinger IB, Simpson SA, Nowak NJ, Small KW, Maumenee IH, Rosenfeld PJ, Sieving PA, Shows TB, Ayyagari R. Bestrophin gene mutations in patients with Best vitelliform macular dystrophy. Genomics. 1999;58:98–101. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

