Identification of severe acute respiratory syndrome coronavirus replicase products and characterization of papain-like protease activity
- PMID: 15564471
- PMCID: PMC533933
- DOI: 10.1128/JVI.78.24.13600-13612.2004
Identification of severe acute respiratory syndrome coronavirus replicase products and characterization of papain-like protease activity
Abstract
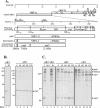
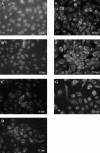
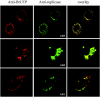
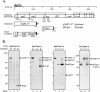
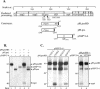
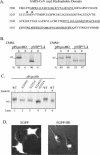
Gene 1 of the coronavirus associated with severe acute respiratory syndrome (SARS) encodes replicase polyproteins that are predicted to be processed into 16 nonstructural proteins (nsps 1 to 16) by two viral proteases, a papain-like protease (PLpro) and a 3C-like protease (3CLpro). Here, we identify SARS coronavirus amino-terminal replicase products nsp1, nsp2, and nsp3 and describe trans-cleavage assays that characterize the protease activity required to generate these products. We generated polyclonal antisera to glutathione S-transferase-replicase fusion proteins and used the antisera to detect replicase intermediates and products in pulse-chase experiments. We found that nsp1 (p20) is rapidly processed from the replicase polyprotein. In contrast, processing at the nsp2/3 site is less efficient, since a approximately 300-kDa intermediate (NSP2-3) is detected, but ultimately nsp2 (p71) and nsp3 (p213) are generated. We found that SARS coronavirus replicase products can be detected by 4 h postinfection in the cytoplasm of infected cells and that nsps 1 to 3 colocalize with newly synthesized viral RNA in punctate, perinuclear sites consistent with their predicted role in viral RNA synthesis. To determine if PLpro is responsible for processing these products, we cloned and expressed the PLpro domain and the predicted substrates and established PLpro trans-cleavage assays. We found that the PLpro domain is sufficient for processing the predicted nsp1/2 and nsp2/3 sites. Interestingly, expression of an extended region of PLpro that includes the downstream hydrophobic domain was required for processing at the predicted nsp3/4 site. We found that the hydrophobic domain is inserted into membranes and that the lumenal domain is glycosylated at asparagine residues 2249 and 2252. Thus, the hydrophobic domain may anchor the replication complex to intracellular membranes. These studies revealed that PLpro can cleave in trans at the three predicted cleavage sites and that it requires membrane association to process the nsp3/4 cleavage site.
Figures
References
-
- Anand, K., J. Ziebuhr, P. Wadhwani, J. R. Mesters, and R. Hilgenfeld. 2003. Coronavirus main proteinase (3CLpro) structure: basis for design of anti-SARS drugs. Science 300:1763-1767. - PubMed
-
- Bonilla, P. J., S. A. Hughes, J. D. Pinon, and S. R. Weiss. 1995. Characterization of the leader papain-like proteinase of MHV-A59: identification of a new in vitro cleavage site. Virology 209:489-497. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

