Protein kinase C-alpha activity is required for respiratory syncytial virus fusion to human bronchial epithelial cells
- PMID: 15564481
- PMCID: PMC533893
- DOI: 10.1128/JVI.78.24.13717-13726.2004
Protein kinase C-alpha activity is required for respiratory syncytial virus fusion to human bronchial epithelial cells
Abstract
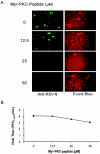
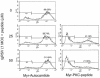
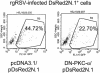
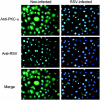
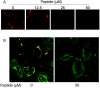
Respiratory syncytial virus (RSV) infection activates protein kinase C (PKC), but the precise PKC isoform(s) involved and its role(s) remain to be elucidated. On the basis of the activation kinetics of different signaling pathways and the effect of various PKC inhibitors, it was reasoned that PKC activation is important in the early stages of RSV infection, especially RSV fusion and/or replication. Herein, the role of PKC-alpha during the early stages of RSV infection in normal human bronchial epithelial cells is determined. The results show that the blocking of PKC-alpha activation by classical inhibitors, pseudosubstrate peptides, or the overexpression of dominant-negative mutants of PKC-alpha in these cells leads to significantly decreased RSV infection. RSV induces phosphorylation, activation, and cytoplasm-to-membrane translocation of PKC-alpha. Also, PKC-alpha colocalizes with virus particles and is required for RSV fusion to the cell membrane. Thus, PKC-alpha could provide a new pharmacological target for controlling RSV infection.
Figures
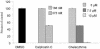



References
-
- Bitko, V., A. Velazquez, L. Yang, Y.-C. Yang, and S. Barik. 1997. Transcriptional induction of multiple cytokines by human respiratory syncytial virus requires activation of NF-κB and is inhibited by sodium salicylate and aspirin. Virology 232:369-378. - PubMed
-
- Brown, G., J. Aitken, H. W. Rixon, and R. J. Sugrue. 2002. Caveolin-1 is incorporated into mature respiratory syncytial virus particles during virus assembly on the surface of virus-infected cells. J. Gen. Virol. 83:611-621. - PubMed
-
- Brown, G., H. W. Rixon, and R. J. Sugrue. 2002. Respiratory syncytial virus assembly occurs in GM1-rich regions of the host-cell membrane and alters the cellular distribution of tyrosine phosphorylated caveolin-1. J. Gen. Virol. 83:1841-1850. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

