Cyclin/CDK regulates the nucleocytoplasmic localization of the human papillomavirus E1 DNA helicase
- PMID: 15564503
- PMCID: PMC533924
- DOI: 10.1128/JVI.78.24.13954-13965.2004
Cyclin/CDK regulates the nucleocytoplasmic localization of the human papillomavirus E1 DNA helicase
Abstract
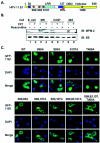
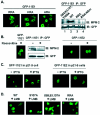
Cyclin-dependent kinases (CDKs) play key roles in eukaryotic DNA replication and cell cycle progression. Phosphorylation of components of the preinitiation complex activates replication and prevents reinitiation. One mechanism is mediated by nuclear export of critical proteins. Human papillomavirus (HPV) DNA replication requires cellular machinery in addition to the viral replicative DNA helicase E1 and origin recognition protein E2. E1 phosphorylation by cyclin/CDK is critical for efficient viral DNA replication. We now show that E1 is phosphorylated by CDKs in vivo and that phosphorylation regulates its nucleocytoplasmic localization. We identified a conserved regulatory region for localization which contains a dominant leucine-rich nuclear export sequence (NES), the previously defined cyclin binding motif, three serine residues that are CDK substrates, and a putative bipartite nuclear localization sequence. We show that E1 is exported from the nucleus by a CRM1-dependent mechanism unless the NES is inactivated by CDK phosphorylation. Replication activities of E1 phosphorylation site mutations are reduced and correlate inversely with their increased cytoplasmic localization. Nuclear localization and replication activities of most of these mutations are enhanced or restored by mutations in the NES. Collectively, our data demonstrate that CDK phosphorylation controls E1 nuclear localization to support viral DNA amplification. Thus, HPV adopts and adapts the cellular regulatory mechanism to complete its reproductive program.
Figures


References
-
- Abraham, R. T. 2001. Cell cycle checkpoint signaling through the ATM and ATR kinases. Genes Dev. 15:2177-2196. - PubMed
-
- Amin, A. A., S. Titolo, A. Pelletier, D. Fink, M. G. Cordingley, and J. Archambault. 2000. Identification of domains of the HPV11 E1 protein required for DNA replication in vitro. Virology 272:137-150. - PubMed
-
- Auster, A. S., and L. Joshua-Tor. 2004. The DNA-binding domain of human papillomavirus type 18 E1: crystal structure, dimerization, and DNA binding. J. Biol. Chem. 279:3733-3742. - PubMed
-
- Bell, S. P., and A. Dutta. 2002. DNA replication in eukaryotic cells. Annu. Rev. Biochem. 71:333-374. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

