Overexpression of 7a, a protein specifically encoded by the severe acute respiratory syndrome coronavirus, induces apoptosis via a caspase-dependent pathway
- PMID: 15564512
- PMCID: PMC533950
- DOI: 10.1128/JVI.78.24.14043-14047.2004
Overexpression of 7a, a protein specifically encoded by the severe acute respiratory syndrome coronavirus, induces apoptosis via a caspase-dependent pathway
Abstract
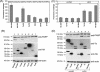
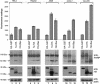
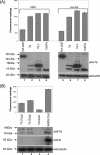
Besides genes that are homologous to proteins found in other coronaviruses, the severe acute respiratory syndrome coronavirus genome also contains nine other potential open reading frames. Previously, we have characterized the expression and cellular localization of two of these "accessory" viral proteins, 3a (previously termed U274) and 7a (previously termed U122). In this study, we further examined whether they can induce apoptosis, which has been observed clinically. We showed that the overexpression of 7a, but not of 3a or the viral structural proteins, nucleocapsid, membrane, and envelope, induces apoptosis. 7a induces apoptosis via a caspase-dependent pathway and in cell lines derived from different organs, including lung, kidney, and liver.
Figures
Similar articles
-
A novel severe acute respiratory syndrome coronavirus protein, U274, is transported to the cell surface and undergoes endocytosis.J Virol. 2004 Jul;78(13):6723-34. doi: 10.1128/JVI.78.13.6723-6734.2004. J Virol. 2004. PMID: 15194747 Free PMC article.
-
SARS coronavirus 7a protein blocks cell cycle progression at G0/G1 phase via the cyclin D3/pRb pathway.Virology. 2006 Mar 1;346(1):74-85. doi: 10.1016/j.virol.2005.10.015. Epub 2005 Nov 21. Virology. 2006. PMID: 16303160 Free PMC article.
-
Characterization of a unique group-specific protein (U122) of the severe acute respiratory syndrome coronavirus.J Virol. 2004 Jul;78(14):7311-8. doi: 10.1128/JVI.78.14.7311-7318.2004. J Virol. 2004. PMID: 15220404 Free PMC article.
-
Characterization of viral proteins encoded by the SARS-coronavirus genome.Antiviral Res. 2005 Feb;65(2):69-78. doi: 10.1016/j.antiviral.2004.10.001. Antiviral Res. 2005. PMID: 15708633 Free PMC article. Review.
-
Understanding the accessory viral proteins unique to the severe acute respiratory syndrome (SARS) coronavirus.Antiviral Res. 2006 Nov;72(2):78-88. doi: 10.1016/j.antiviral.2006.05.010. Epub 2006 Jun 6. Antiviral Res. 2006. PMID: 16820226 Free PMC article. Review.
Cited by
-
Comparative highlights on MERS-CoV, SARS-CoV-1, SARS-CoV-2, and NEO-CoV.EXCLI J. 2022 Sep 29;21:1245-1272. doi: 10.17179/excli2022-5355. eCollection 2022. EXCLI J. 2022. PMID: 36483910 Free PMC article. Review.
-
Severe acute respiratory syndrome and coronavirus.Infect Dis Clin North Am. 2010 Sep;24(3):619-38. doi: 10.1016/j.idc.2010.04.009. Infect Dis Clin North Am. 2010. PMID: 20674795 Free PMC article. Review.
-
Severe acute respiratory syndrome coronavirus gene 7 products contribute to virus-induced apoptosis.J Virol. 2007 Oct;81(20):11054-68. doi: 10.1128/JVI.01266-07. Epub 2007 Aug 8. J Virol. 2007. PMID: 17686858 Free PMC article.
-
Cell cycle arrest and apoptosis induced by the coronavirus infectious bronchitis virus in the absence of p53.Virology. 2007 Sep 1;365(2):435-45. doi: 10.1016/j.virol.2007.04.015. Epub 2007 May 9. Virology. 2007. PMID: 17493653 Free PMC article.
-
The 7a accessory protein of severe acute respiratory syndrome coronavirus acts as an RNA silencing suppressor.J Virol. 2010 Oct;84(19):10395-401. doi: 10.1128/JVI.00748-10. Epub 2010 Jul 14. J Virol. 2010. PMID: 20631126 Free PMC article.
References
-
- Barber, G. N. 2001. Host defense, viruses and apoptosis. Cell Death Differ. 8:113-126. - PubMed
-
- Benedict, C. A., P. S. Norris, and C. F. Ware. 2002. To kill or be killed: viral evasion of apoptosis. Nat. Immunol. 3:1013-1018. - PubMed
-
- Brown, T. D. K., and I. Brierly. 1995. The coronavirus nonstructural proteins, p. 191-217. In S. G. Siddell (ed.), The Coronaviridae. Plenum Press, New York, N.Y.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

