Brain-derived neurotrophic factor regulation of retinal growth cone filopodial dynamics is mediated through actin depolymerizing factor/cofilin
- PMID: 15564592
- PMCID: PMC6730129
- DOI: 10.1523/JNEUROSCI.2836-04.2004
Brain-derived neurotrophic factor regulation of retinal growth cone filopodial dynamics is mediated through actin depolymerizing factor/cofilin
Abstract
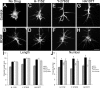
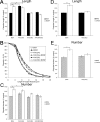
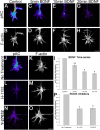
The molecular mechanisms by which neurotrophins regulate growth cone motility are not well understood. This study investigated the signaling involved in transducing BDNF-induced increases of filopodial dynamics. Our results indicate that BDNF regulates filopodial length and number through a Rho kinase-dependent mechanism. Additionally, actin depolymerizing factor (ADF)/cofilin activity is necessary and sufficient to transduce the effects of BDNF. Our data indicate that activation of ADF/cofilin mimics the effects of BDNF on filopodial dynamics, whereas ADF/cofilin inactivity blocks the effects of BDNF. Furthermore, BDNF promotes the activation of ADF/cofilin by reducing the phosphorylation of ADF/cofilin. Although inhibition of myosin II also enhances filopodial length, our results indicate that BDNF signaling is independent of myosin II activity and that the two pathways result in additive effects on filopodial length. Thus, filopodial extension is regulated by at least two independent mechanisms. The BDNF-dependent pathway works via regulation of ADF/cofilin, independently of myosin II activity.
Figures

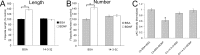
Similar articles
-
Cdc42 participates in the regulation of ADF/cofilin and retinal growth cone filopodia by brain derived neurotrophic factor.J Neurobiol. 2006 Feb 5;66(2):103-14. doi: 10.1002/neu.20204. J Neurobiol. 2006. PMID: 16215999
-
Regulating filopodial dynamics through actin-depolymerizing factor/cofilin.Anat Sci Int. 2004 Dec;79(4):173-83. doi: 10.1111/j.1447-073x.2004.00087.x. Anat Sci Int. 2004. PMID: 15633455 Review.
-
Regulation of growth cone actin dynamics by ADF/cofilin.J Histochem Cytochem. 2003 Apr;51(4):411-20. doi: 10.1177/002215540305100402. J Histochem Cytochem. 2003. PMID: 12642619 Review.
-
p75 neurotrophin receptor signaling regulates growth cone filopodial dynamics through modulating RhoA activity.J Neurosci. 2004 May 5;24(18):4363-72. doi: 10.1523/JNEUROSCI.0404-04.2004. J Neurosci. 2004. PMID: 15128850 Free PMC article.
-
Regulating actin dynamics in neuronal growth cones by ADF/cofilin and rho family GTPases.J Neurobiol. 2000 Aug;44(2):126-44. J Neurobiol. 2000. PMID: 10934317 Review.
Cited by
-
Changes in synaptic morphology accompany actin signaling during LTP.J Neurosci. 2007 May 16;27(20):5363-72. doi: 10.1523/JNEUROSCI.0164-07.2007. J Neurosci. 2007. PMID: 17507558 Free PMC article.
-
Development of the vagal innervation of the gut: steering the wandering nerve.Neurogastroenterol Motil. 2011 Oct;23(10):898-911. doi: 10.1111/j.1365-2982.2011.01764.x. Epub 2011 Aug 18. Neurogastroenterol Motil. 2011. PMID: 21851506 Free PMC article. Review.
-
Cofilin 1 activation prevents the defects in axon elongation and guidance induced by extracellular alpha-synuclein.Sci Rep. 2015 Nov 12;5:16524. doi: 10.1038/srep16524. Sci Rep. 2015. PMID: 26558842 Free PMC article.
-
BDNF signaling during the lifetime of dendritic spines.Cell Tissue Res. 2020 Oct;382(1):185-199. doi: 10.1007/s00441-020-03226-5. Epub 2020 Jun 14. Cell Tissue Res. 2020. PMID: 32537724 Free PMC article. Review.
-
Axonal neuropathy-associated TRPV4 regulates neurotrophic factor-derived axonal growth.J Biol Chem. 2012 Feb 17;287(8):6014-24. doi: 10.1074/jbc.M111.316315. Epub 2011 Dec 20. J Biol Chem. 2012. PMID: 22187434 Free PMC article.
References
-
- Agnew BJ, Minamide LS, Bamburg JR (1995) Reactivation of phosphorylated actin depolymerizing factor and identification of the regulatory site. J Biol Chem 270: 17582-17587. - PubMed
-
- Aizawa H, Wakatsuki S, Ishii A, Moriyama K, Sasaki Y, Ohashi K, Sekine-Aizawa Y, Sehara-Fujisawa A, Mizuno K, Goshima Y, Yahara I (2001) Phosphorylation of cofilin by LIM-kinase is necessary for semaphorin 3A-induced growth cone collapse. Nat Neurosci 4: 367-373. - PubMed
-
- Alsina B, Vu T, Cohen-Cory S (2001) Visualizing synapse formation in arborizing optic axons in vivo: dynamics and modulation by BDNF. Nat Neurosci 4: 1093-1101. - PubMed
-
- Amano M, Ito M, Kimura K, Fukata Y, Chihara K, Nakano T, Matsuura Y, Kaibuchi K (1996) Phosphorylation and activation of myosin by Rho-associated kinase (Rho-kinase). J Biol Chem 271: 20246-20249. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
