Molecular mechanism of voltage sensor movements in a potassium channel
- PMID: 15565171
- PMCID: PMC535096
- DOI: 10.1038/sj.emboj.7600484
Molecular mechanism of voltage sensor movements in a potassium channel
Abstract
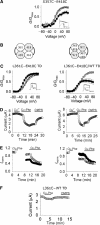
Voltage-gated potassium channels are six-transmembrane (S1-S6) proteins that form a central pore domain (4 x S5-S6) surrounded by four voltage sensor domains (S1-S4), which detect changes in membrane voltage and control pore opening. Upon depolarization, the S4 segments move outward carrying charged residues across the membrane field, thereby leading to the opening of the pore. The mechanism of S4 motion is controversial. We have investigated how S4 moves relative to the pore domain in the prototypical Shaker potassium channel. We introduced pairs of cysteines, one in S4 and the other in S5, and examined proximity changes between each pair of cysteines during activation, using Cd2+ and copper-phenanthroline, which crosslink the cysteines with metal and disulphide bridges, respectively. Modelling of the results suggests a novel mechanism: in the resting state, the top of the S3b-S4 voltage sensor paddle lies close to the top of S5 of the adjacent subunit, but moves towards the top of S5 of its own subunit during depolarization--this motion is accompanied by a reorientation of S4 charges to the extracellular phase.
Figures



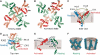
References
-
- Aggarwal SK, MacKinnon R (1996) Contribution of the S4 segment to gating charge in the Shaker K+ channel. Neuron 16: 1169–1177 - PubMed
-
- Ahern CA, Horn R (2004b) Stirring up controversy with a voltage sensor paddle. Trends Neurosci 27: 303–307 - PubMed
-
- Aziz QH, Partridge CJ, Munsey TS, Sivaprasadarao A (2002) Depolarization induces intersubunit cross-linking in a S4 cysteine mutant of the Shaker potassium channel. J Biol Chem 277: 42719–42725 - PubMed
-
- Baker OS, Larsson HP, Mannuzzu LM, Isacoff EY (1998) Three transmembrane conformations and sequence-dependent displacement of the S4 domain in Shaker K+ channel gating. Neuron 20: 1283–1294 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources

