MSK1 activity is controlled by multiple phosphorylation sites
- PMID: 15568999
- PMCID: PMC1134980
- DOI: 10.1042/BJ20041501
MSK1 activity is controlled by multiple phosphorylation sites
Abstract
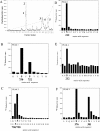
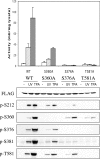
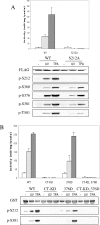
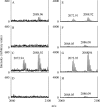
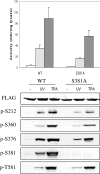
MSK1 (mitogen- and stress-activated protein kinase) is a kinase activated in cells downstream of both the ERK1/2 (extracellular-signal-regulated kinase) and p38 MAPK (mitogen-activated protein kinase) cascades. In the present study, we show that, in addition to being phosphorylated on Thr-581 and Ser-360 by ERK1/2 or p38, MSK1 can autophosphorylate on at least six sites: Ser-212, Ser-376, Ser-381, Ser-750, Ser-752 and Ser-758. Of these sites, the N-terminal T-loop residue Ser-212 and the 'hydrophobic motif' Ser-376 are phosphorylated by the C-terminal kinase domain of MSK1, and their phosphorylation is essential for the catalytic activity of the N-terminal kinase domain of MSK1 and therefore for the phosphorylation of MSK1 substrates in vitro. Ser-381 is also phosphorylated by the C-terminal kinase domain, and mutation of Ser-381 decreases MSK1 activity, probably through the inhibition of Ser-376 phosphorylation. Ser-750, Ser-752 and Ser-758 are phosphorylated by the N-terminal kinase domain; however, their function is not known. The activation of MSK1 in cells therefore requires the activation of the ERK1/2 or p38 MAPK cascades and does not appear to require additional signalling inputs. This is in contrast with the closely related RSK (p90 ribosomal S6 kinase) proteins, whose activity requires phosphorylation by PDK1 (3-phosphoinositide-dependent protein kinase 1) in addition to phosphorylation by ERK1/2.
Figures



References
-
- Pierrat B., Correia J. d. S., Mary J.-L., Tomas-Zuber M., Lesslauer W. RSK-B, a novel ribosomal S6 kinase family member, is a CREB kinase under dominant control of p38alpha mitogen-activated protein kinase (p38alpha MAPK) J. Biol. Chem. 1998;273:29661–29671. - PubMed
-
- New L., Zhao M., Li Y., Bassett W. W., Feng Y., Ludwig S., Padova F. D., Gram H., Han J. Cloning and characterization of RLPK, a novel RSK-related protein kinase. J. Biol. Chem. 1999;274:1026–1032. - PubMed
-
- Arthur J. S. C., Cohen P. MSK1 is required for CREB phosphorylation in response to mitogens in mouse embryonic stem cells. FEBS lett. 2000;482:44–48. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

