Covert operations of uropathogenic Escherichia coli within the urinary tract
- PMID: 15569242
- PMCID: PMC2523259
- DOI: 10.1111/j.1600-0854.2004.00251.x
Covert operations of uropathogenic Escherichia coli within the urinary tract
Abstract
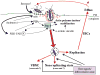
Entry into host cells is required for many bacterial pathogens to effectively disseminate within a host, avoid immune detection and cause disease. In recent years, many ostensibly extracellular bacteria have been shown to act as opportunistic intracellular pathogens. Among these are strains of uropathogenic Escherichia coli (UPEC), the primary causative agents of urinary tract infections (UTIs). UPEC are able to transiently invade, survive and multiply within the host cells and tissues constituting the urinary tract. Invasion of host cells by UPEC is promoted independently by distinct virulence factors, including cytotoxic necrotizing factor, Afa/Dr adhesins, and type 1 pili. Here we review the diverse mechanisms and consequences of host cell invasion by UPEC, focusing also on the impact of these processes on the persistence and recurrence of UTIs.
Figures



References
-
- Janeway CA, Travers P, Walport M, Capra JD. The Immune System in Health and Disease. 4. New York: Garland; 1999. Immunobiology.
-
- Osterlund A, Engstrand L. Intracellular penetration and survival of Streptococcus pyogenes in respiratory epithelial cells in vitro. Acta Otolaryngol. 1995;115:685–688. - PubMed
-
- Petersen AM, Krogfelt KA. Helicobacter pylori: an invading micro-organism? A review FEMS. Immunol Med Microbiol. 2003;36:117–126. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

