Aging activates adipogenic and suppresses osteogenic programs in mesenchymal marrow stroma/stem cells: the role of PPAR-gamma2 transcription factor and TGF-beta/BMP signaling pathways
- PMID: 15569355
- PMCID: PMC1850101
- DOI: 10.1111/j.1474-9728.2004.00127.x
Aging activates adipogenic and suppresses osteogenic programs in mesenchymal marrow stroma/stem cells: the role of PPAR-gamma2 transcription factor and TGF-beta/BMP signaling pathways
Abstract
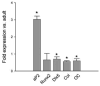
Osteoblasts and adipocytes originate from a common progenitor, which arises from bone marrow mesenchymal stroma/stem cells (mMSC). Aging causes a decrease in the number of bone-forming osteoblasts and an increase in the number of marrow adipocytes. Here, we demonstrate that, during aging, the status of mMSC changes with respect to both their intrinsic differentiation potential and production of signaling molecules, which contributes to the formation of a specific marrow microenvironment necessary for maintenance of bone homeostasis. Aging causes a decrease in the commitment of mMSC to the osteoblast lineage and an increase in the commitment to the adipocyte lineage. This is reflected by changes in the expression of phenotype-specific gene markers. The expression of osteoblast-specific transcription factors, Runx2 and Dlx5, and osteoblast markers, collagen and osteocalcin, is decreased in aged mMSC. Conversely, the expression of adipocyte-specific transcription factor PPAR-gamma2, shown previously to regulate osteoblast development and bone formation negatively and to regulate marrow adipocyte differentiation positively, is increased, as is a gene marker of adipocyte phenotype, fatty acid binding protein aP2. Furthermore, production of an endogenous PPAR-gamma activator(s) that stimulates adipocyte differentiation and production of autocrine/paracrine factor(s) that suppresses the osteoblastic phenotype are also increased. In addition, expression of different components of TGF-beta and BMP2/4 signaling pathways is altered, suggesting that activities of these two cytokines essential for bone homeostasis change with aging.
Figures

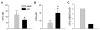
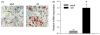
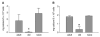
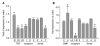
References
-
- Abe E, Yamamoto M, Taguchi Y, Lecka-Czernik B, O’Brian CA, Economides AN, Stahl N, Jilka RL, Manolagas SC. Essential requirement of BMPs-2/4 for both osteoblast and osteoclast formation in murine bone marrow cultures from adult mice: antagonism by noggin. J Bone Miner Res. 2000;15:663–673. - PubMed
-
- Banerjee C, Javed A, Choi JY, Green J, Rosen V, van Wijnen AJ, Stein JL, Lian JB, Stein GS. Differential regulation of the two principal Runx2/Cbfa1 n-terminal isoforms in response to bone morphogenetic protein-2 during development of the osteoblast phenotype. Endocrinology. 2001;142:4026–4039. - PubMed
-
- Bianco P, Riminucci M, Gronthos S, Robey PG. Bone marrow stromal stem cells: nature, biology, and potential applications. Stem Cells. 2001;19:180–192. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

