Progenitor cells of the testosterone-producing Leydig cells revealed
- PMID: 15569711
- PMCID: PMC2172461
- DOI: 10.1083/jcb.200409107
Progenitor cells of the testosterone-producing Leydig cells revealed
Abstract
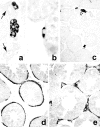
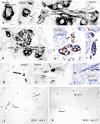
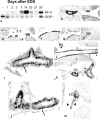
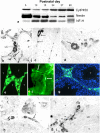
The cells responsible for production of the male sex hormone testosterone, the Leydig cells of the testis, are post-mitotic cells with neuroendocrine characteristics. Their origin during ontogeny and regeneration processes is still a matter of debate. Here, we show that cells of testicular blood vessels, namely vascular smooth muscle cells and pericytes, are the progenitors of Leydig cells. Resembling stem cells of the nervous system, the Leydig cell progenitors are characterized by the expression of nestin. Using an in vivo model to induce and monitor the synchronized generation of a completely new Leydig cell population in adult rats, we demonstrate specific proliferation of vascular progenitors and their subsequent transdifferentiation into steroidogenic Leydig cells which, in addition, rapidly acquire neuronal and glial properties. These findings, shown to be representative also for ontogenetic Leydig cell formation and for the human testis, provide further evidence that cellular components of blood vessels can act as progenitor cells for organogenesis and repair.
Figures

References
-
- Alliot, F., J. Rutin, P.J.M. Leenen, and B. Pessac. 1999. Pericytes and periendothelial cells of brain parenchyma vessels co-express aminopeptidase N, aminopeptidase A, and nestin. J. Neurosci. Res. 58:367–378. - PubMed
-
- Allt, G., and J.G. Lawrenson. 2001. Pericytes: cell biology and pathology. Cells Tissues Organs. 169:1–11. - PubMed
-
- Ariyaratne, H.B.S., S.M.L.C. Mendis-Handagama, D.B. Hales, and J.I. Mason. 2000. Studies on the onset of Leydig precursor cell differentiation in the prepubertal rat testis. Biol. Reprod. 63:165–171. - PubMed
-
- Basciani, S., S. Mariani, M. Arizzi, S. Ulisse, N. Rucci, E.A. Jannini, C.D. Rocca, A. Manicone, C. Carani, G. Spera, and L. Gnessi. 2002. Expression of platelet-derived growth factor-A (PDGF-A), PDGF-B, and PDGF receptor-α and -β during human testicular development and disease. J. Clin. Endocrinol. Metab. 87:2310–2319. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

