Phosphate deprivation induces transfer of DGDG galactolipid from chloroplast to mitochondria
- PMID: 15569715
- PMCID: PMC2172463
- DOI: 10.1083/jcb.200407022
Phosphate deprivation induces transfer of DGDG galactolipid from chloroplast to mitochondria
Abstract
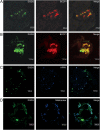
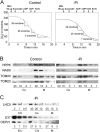
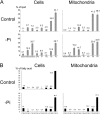
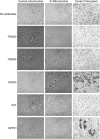
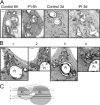
In many soils plants have to grow in a shortage of phosphate, leading to development of phosphate-saving mechanisms. At the cellular level, these mechanisms include conversion of phospholipids into glycolipids, mainly digalactosyldiacylglycerol (DGDG). The lipid changes are not restricted to plastid membranes where DGDG is synthesized and resides under normal conditions. In plant cells deprived of phosphate, mitochondria contain a high concentration of DGDG, whereas mitochondria have no glycolipids in control cells. Mitochondria do not synthesize this pool of DGDG, which structure is shown to be characteristic of a DGD type enzyme present in plastid envelope. The transfer of DGDG between plastid and mitochondria is investigated and detected between mitochondria-closely associated envelope vesicles and mitochondria. This transfer does not apparently involve the endomembrane system and would rather be dependent upon contacts between plastids and mitochondria. Contacts sites are favored at early stages of phosphate deprivation when DGDG cell content is just starting to respond to phosphate deprivation.
Figures

References
-
- Achleitner, G., B. Gaigg, A. Krasser, E. Kainersdorfer, S.D. Kohlwein, A. Perktold, G. Zellnig, and G. Daum. 1999. Association between the endoplasmic reticulum and mitochondria of yeast facilitates interorganelle transport of phospholipids through membrane contact. Eur. J. Biochem. 264:545–553. - PubMed
-
- Andersson, M.X., M.H. Stridh, K.E. Larsson, C. Liljenberg, and A.S. Sandelius. 2003. Phosphate-deficient oat replaces a major portion of the plasma membrane phospholipids with the galactolipid digalactosyldiacylglycerol. FEBS Lett. 537:128–132. - PubMed
-
- Ardail, D., F. Lerme, and P. Louisot. 1991. Involvement of contact sites in phosphatidylserine import into liver mitochondria. J. Biol. Chem. 266:7978–7981. - PubMed
-
- Awai, K., E. Maréchal, M.A. Block, D. Brun, T. Masuda, H. Shimada, K. Takamiya, H. Ohta, and J. Joyard. 2001. Two types of MGDG synthase genes, found widely in both 16:3 and 18:3 plants, differentially mediate galactolipid syntheses in photosynthetic and nonphotosynthetic tissues in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA. 98:10960–10965. - PMC - PubMed

