HIV-1 Nef disrupts MHC-I trafficking by recruiting AP-1 to the MHC-I cytoplasmic tail
- PMID: 15569716
- PMCID: PMC2172469
- DOI: 10.1083/jcb.200407031
HIV-1 Nef disrupts MHC-I trafficking by recruiting AP-1 to the MHC-I cytoplasmic tail
Abstract
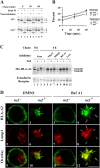
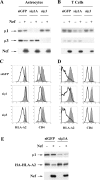
To avoid immune recognition by cytotoxic T lymphocytes (CTLs), human immunodeficiency virus (HIV)-1 Nef disrupts the transport of major histocompatibility complex class I molecules (MHC-I) to the cell surface in HIV-infected T cells. However, the mechanism by which Nef does this is unknown. We report that Nef disrupts MHC-I trafficking by rerouting newly synthesized MHC-I from the trans-Golgi network (TGN) to lysosomal compartments for degradation. The ability of Nef to target MHC-I from the TGN to lysosomes is dependent on expression of the mu1 subunit of adaptor protein (AP) AP-1A, a cellular protein complex implicated in TGN to endolysosomal pathways. We demonstrate that in HIV-infected primary T cells, Nef promotes a physical interaction between endogenous AP-1 and MHC-I. Moreover, we present data that this interaction uses a novel AP-1 binding site that requires amino acids in the MHC-I cytoplasmic tail. In sum, our evidence suggests that binding of AP-1 to the Nef-MHC-I complex is an important step required for inhibition of antigen presentation by HIV.
Figures

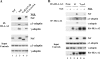



Similar articles
-
Two Functional Variants of AP-1 Complexes Composed of either γ2 or γ1 Subunits Are Independently Required for Major Histocompatibility Complex Class I Downregulation by HIV-1 Nef.J Virol. 2020 Mar 17;94(7):e02039-19. doi: 10.1128/JVI.02039-19. Print 2020 Mar 17. J Virol. 2020. PMID: 31915283 Free PMC article.
-
ADP ribosylation factor 1 activity is required to recruit AP-1 to the major histocompatibility complex class I (MHC-I) cytoplasmic tail and disrupt MHC-I trafficking in HIV-1-infected primary T cells.J Virol. 2011 Dec;85(23):12216-26. doi: 10.1128/JVI.00056-11. Epub 2011 Sep 14. J Virol. 2011. PMID: 21917951 Free PMC article.
-
The tyrosine binding pocket in the adaptor protein 1 (AP-1) mu1 subunit is necessary for Nef to recruit AP-1 to the major histocompatibility complex class I cytoplasmic tail.J Biol Chem. 2008 Feb 8;283(6):3011-3022. doi: 10.1074/jbc.M707760200. Epub 2007 Dec 11. J Biol Chem. 2008. PMID: 18073204
-
HIV immune evasion disruption of antigen presentation by the HIV Nef protein.Adv Virus Res. 2011;80:103-27. doi: 10.1016/B978-0-12-385987-7.00005-1. Adv Virus Res. 2011. PMID: 21762823 Free PMC article. Review.
-
HIV and SIV Nef modulate signal transduction and protein sorting in T cells.Cold Spring Harb Symp Quant Biol. 1999;64:453-63. doi: 10.1101/sqb.1999.64.453. Cold Spring Harb Symp Quant Biol. 1999. PMID: 11232322 Review. No abstract available.
Cited by
-
Viral Interactions with Adaptor-Protein Complexes: A Ubiquitous Trait among Viral Species.Int J Mol Sci. 2021 May 17;22(10):5274. doi: 10.3390/ijms22105274. Int J Mol Sci. 2021. PMID: 34067854 Free PMC article. Review.
-
Downregulation of CD4 by human immunodeficiency virus type 1 Nef is dependent on clathrin and involves direct interaction of Nef with the AP2 clathrin adaptor.J Virol. 2007 Apr;81(8):3877-90. doi: 10.1128/JVI.02725-06. Epub 2007 Jan 31. J Virol. 2007. PMID: 17267500 Free PMC article.
-
AP-2 Adaptor Complex-Dependent Enhancement of HIV-1 Replication by Nef in the Absence of the Nef/AP-2 Targets SERINC5 and CD4.mBio. 2023 Feb 28;14(1):e0338222. doi: 10.1128/mbio.03382-22. Epub 2023 Jan 9. mBio. 2023. PMID: 36622146 Free PMC article.
-
HIV-1 Nef employs two distinct mechanisms to modulate Lck subcellular localization and TCR induced actin remodeling.PLoS One. 2007 Nov 21;2(11):e1212. doi: 10.1371/journal.pone.0001212. PLoS One. 2007. PMID: 18030346 Free PMC article.
-
HIV-1 Nef disrupts intracellular trafficking of major histocompatibility complex class I, CD4, CD8, and CD28 by distinct pathways that share common elements.J Virol. 2011 Jul;85(14):6867-81. doi: 10.1128/JVI.00229-11. Epub 2011 May 4. J Virol. 2011. PMID: 21543478 Free PMC article.
References
-
- Aiken, C., J. Konner, N.R. Landau, M.E. Lenburg, and D. Trono. 1994. Nef induces CD4 endocytosis: requirement for a critical dileucine motif in the membrane-proximal CD4 cytoplasmic domain. Cell. 76:853–864. - PubMed
-
- Alexander, M., Y.C. Bor, K.S. Ravichandran, M.L. Hammarskjold, and D. Rekosh. 2004. Human immunodeficiency virus type 1 Nef associates with lipid rafts to downmodulate cell surface CD4 and class I major histocompatibility complex expression and to increase viral infectivity. J. Virol. 78:1685–1696. - PMC - PubMed
-
- Blagoveshchenskaya, A.D., L. Thomas, S.F. Feliciangeli, C.H. Hung, and G. Thomas. 2002. HIV-1 Nef downregulates MHC-I by a PACS-1- and PI3K-regulated ARF6 endocytic pathway. Cell. 111:853–866. - PubMed
-
- Bonifacino, J.S., and L.M. Traub. 2003. Signals for sorting of transmembrane proteins to endosomes and lysosomes. Annu. Rev. Biochem. 72:395–447. - PubMed
-
- Bresnahan, P.A., W. Yonemoto, S. Ferrell, D. Williams-Herman, R. Geleziunas, and W.C. Greene. 1998. A dileucine motif in HIV-1 Nef acts as an internalization signal for CD4 downregulation and binds the AP-1 clathrin adaptor. Curr. Biol. 8:1235–1238. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

