Inhibitory effect of palmitate on the mitochondrial NADH:ubiquinone oxidoreductase (complex I) as related to the active-de-active enzyme transition
- PMID: 15571492
- PMCID: PMC1134997
- DOI: 10.1042/BJ20041703
Inhibitory effect of palmitate on the mitochondrial NADH:ubiquinone oxidoreductase (complex I) as related to the active-de-active enzyme transition
Abstract
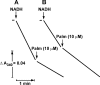
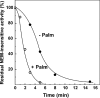
Palmitate rapidly and reversibly inhibits the uncoupled NADH oxidase activity catalysed by activated complex I in inside-out bovine heart submitochondrial particles (IC50 extrapolated to zero enzyme concentration is equal to 9 microM at 25 degrees C, pH 8.0). The NADH:hexa-ammineruthenium reductase activity of complex I is insensitive to palmitate. Partial (approximately 50%) inhibition of the NADH:external quinone reductase activity is seen at saturating palmitate concentration and the residual activity is fully sensitive to piericidin. The uncoupled succinate oxidase activity is considerably less sensitive to palmitate. Only a slight stimulation of tightly coupled respiration with NADH as the substrate is seen at optimal palmitate concentrations, whereas complete relief of the respiratory control is observed with succinate as the substrate. Palmitate prevents the turnover-induced activation of the de-activated complex I (IC50 extrapolated to zero enzyme concentration is equal to 3 microM at 25 degrees C, pH 8.0). The mode of action of palmitate on the NADH oxidase is qualitatively temperature-dependent. Rapid and reversible inhibition of the complex I catalytic activity and its de-active to active state transition are seen at 25 degrees C, whereas the time-dependent irreversible inactivation of the NADH oxidase proceeds at 37 degrees C. Palmitate drastically increases the rate of spontaneous de-activation of complex I in the absence of NADH. Taken together, these results suggest that free fatty acids act as specific complex I-directed inhibitors; at a physiologically relevant temperature (37 degrees C), their inhibitory effects on mitochondrial NADH oxidation is due to perturbation of the pseudo-reversible active-de-active complex I transition.
Figures





References
-
- Carroll J., Shannon R. J., Fearnley I. M., Walker J. E., Hirst J. Definition of the nuclear encoded protein composition of bovine heart mitochondrial Complex I: identification of two new subunits. J. Biol. Chem. 2002;277:50311–50317. - PubMed
-
- Ohnishi T., Magnitsky S., Toulokhonova L., Yano T., Yagi T., Burbaev D. S., Vinogradov A. D. EPR studies of the possible binding sites of the cluster N2, semiquinones, and specific inhibitors of the NADH:quinone oxidoreductase (complex I) Biochem. Soc. Trans. 1999;27:586–591. - PubMed
-
- Weidner U., Geiger S., Ptock A., Friedrich T., Leif H., Weiss H. The gene locus of the proton-translocating NADH:ubiquinone oxidoreductase in Escherichia coli: organization of the 14 genes and relationship between the derived proteins and subunits of mitochondrial complex I. J. Mol. Biol. 1993;233:109–122. - PubMed
-
- Xu X., Matsuno-Yagi A., Yagi T. DNA sequencing of the remaining genes of the gene cluster encoding the energy-transducing NADH-quinone oxidoreductase of Paracoccus denitrificans. Biochemistry. 1993;32:968–981. - PubMed
-
- Dupuis A., Chevallet M., Darrouzet E., Duborjal H., Lunardi J., Issartel J. P. The Complex I from Rhodobacter capsulatus. Biochim. Biophys. Acta. 1998;1364:147–165. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

