Homozygosity for a missense mutation in the 67 kDa isoform of glutamate decarboxylase in a family with autosomal recessive spastic cerebral palsy: parallels with Stiff-Person Syndrome and other movement disorders
- PMID: 15571623
- PMCID: PMC544830
- DOI: 10.1186/1471-2377-4-20
Homozygosity for a missense mutation in the 67 kDa isoform of glutamate decarboxylase in a family with autosomal recessive spastic cerebral palsy: parallels with Stiff-Person Syndrome and other movement disorders
Abstract
Background: Cerebral palsy (CP) is an heterogeneous group of neurological disorders of movement and/or posture, with an estimated incidence of 1 in 1000 live births. Non-progressive forms of symmetrical, spastic CP have been identified, which show a Mendelian autosomal recessive pattern of inheritance. We recently described the mapping of a recessive spastic CP locus to a 5 cM chromosomal region located at 2q24-31.1, in rare consanguineous families.
Methods: Here we present data that refine this locus to a 0.5 cM region, flanked by the microsatellite markers D2S2345 and D2S326. The minimal region contains the candidate gene GAD1, which encodes a glutamate decarboxylase isoform (GAD67), involved in conversion of the amino acid and excitatory neurotransmitter glutamate to the inhibitory neurotransmitter gamma-aminobutyric acid (GABA).
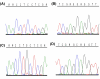
Results: A novel amino acid mis-sense mutation in GAD67 was detected, which segregated with CP in affected individuals.
Conclusions: This result is interesting because auto-antibodies to GAD67 and the more widely studied GAD65 homologue encoded by the GAD2 gene, are described in patients with Stiff-Person Syndrome (SPS), epilepsy, cerebellar ataxia and Batten disease. Further investigation seems merited of the possibility that variation in the GAD1 sequence, potentially affecting glutamate/GABA ratios, may underlie this form of spastic CP, given the presence of anti-GAD antibodies in SPS and the recognised excitotoxicity of glutamate in various contexts.
Figures




References
-
- Eicher PS, Batshaw ML. Cerebral palsy. Pediatric Clinics of North America. 1993;40:537–551. - PubMed
-
- Gustavson KH, Hagberg B, Sanner G. Identical syndromes of cerebral palsy in the same family. Acta Paediatrica Scandinavica. 1969;58:330–340. - PubMed
-
- Volpe J. Neurology of the Newborn. 2. Philadelphia: WB Saunders; 1987.
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

