Role of myosin VIIa and Rab27a in the motility and localization of RPE melanosomes
- PMID: 15572405
- PMCID: PMC2942070
- DOI: 10.1242/jcs.01580
Role of myosin VIIa and Rab27a in the motility and localization of RPE melanosomes
Abstract
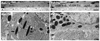
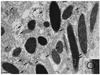
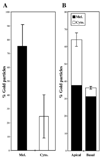
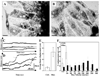
Myosin VIIa functions in the outer retina, and loss of this function causes human blindness in Usher syndrome type 1B (USH1B). In mice with mutant Myo7a, melanosomes in the retinal pigmented epithelium (RPE) are distributed abnormally. In this investigation we detected many proteins in RPE cells that could potentially participate in melanosome transport, but of those tested, only myosin VIIa and Rab27a were found to be required for normal distribution. Two other expressed proteins, melanophilin and myosin Va, both of which are required for normal melanosome distribution in melanocytes, were not required in RPE, despite the association of myosin Va with the RPE melanosome fraction. Both myosin VIIa and myosin Va were immunodetected broadly in sections of the RPE, overlapping with a region of apical filamentous actin. Some 70-80% of the myosin VIIa in RPE cells was detected on melanosome membranes by both subcellular fractionation of RPE cells and quantitative immunoelectron microscopy, consistent with a role for myosin VIIa in melanosome motility. Time-lapse microscopy of melanosomes in primary cultures of mouse RPE cells demonstrated that the melanosomes move in a saltatory manner, interrupting slow movements with short bursts of rapid movement (>1 RR01183m/second). In RPE cells from Myo7a-null mice, both the slow and rapid movements still occurred, except that more melanosomes underwent rapid movements, and each movement extended approximately five times longer (and further). Hence, our studies demonstrate the presence of many potential effectors of melanosome motility and localization in the RPE, with a specific requirement for Rab27a and myosin VIIa, which function by transporting and constraining melanosomes within a region of filamentous actin. The presence of two distinct melanosome velocities in both control and Myo7a-null RPE cells suggests the involvement of at least two motors other than myosin VIIa in melanosome motility, most probably, a microtubule motor and myosin Va.
Figures




References
-
- Avraham KB, Hasson T, Steel KP, Kingsley DM, Russell LB, Mooseker MS, Copeland NG, Jenkins NA. The mouse Snell’s waltzer deafness gene encodes an unconventional myosin required for structural integrity of inner ear hair cells. Nat. Genet. 1995;11:369–375. - PubMed
-
- Back I, Donner KO, Reuter T. The screening effect of the pigment epithelium on the retinal rods in the frog. Vision Res. 1965;5:101–111. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

