Phosphorylation of progesterone receptor serine 400 mediates ligand-independent transcriptional activity in response to activation of cyclin-dependent protein kinase 2
- PMID: 15572662
- PMCID: PMC533997
- DOI: 10.1128/MCB.24.24.10542-10557.2004
Phosphorylation of progesterone receptor serine 400 mediates ligand-independent transcriptional activity in response to activation of cyclin-dependent protein kinase 2
Abstract
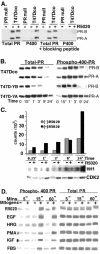
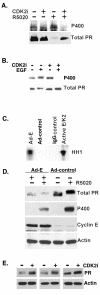
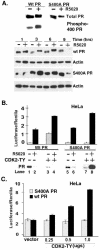
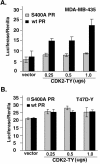
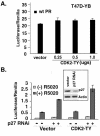
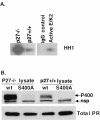
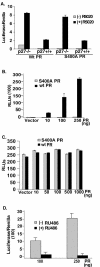
Human progesterone receptors (PR) are phosphorylated by cyclin-dependent protein kinase 2 (CDK2) at multiple sites, including Ser400. Herein, we have addressed the significance of phosphorylation of this residue. PR phospho-Ser400-specific antibodies revealed regulated phosphorylation of Ser400 in response to progestins and mitogens, and this correlated with increased CDK2 levels and activity. Expression of cyclin E elevated CDK2 activity and downregulated PR independently of ligand. Similarly, overexpression of activated mutant CDK2 increased PR transcriptional activity in the absence and presence of progestin. Mutation of PR Ser400 to alanine (S400A) blocked CDK2-induced PR activity in the absence, but not in the presence, of progestin. PR was unresponsive to activated CDK2 in breast cancer cells with elevated p27, and RNA interference knock-down of p27 partially restored CDK2-induced ligand-independent PR activation. Similarly, in p27(-/-) mouse embryonic fibroblasts, elevated CDK2 activity increased wild-type (wt) but not S400A PR transcriptional activity in the absence of progestin. CDK2 induced nuclear localization of unliganded wt but not S400A PR; liganded S400A PR exhibited delayed nuclear accumulation. These studies demonstrate that CDK2 regulates PR in the absence of progestins via phosphorylation of Ser400, thus revealing a novel mechanism for upregulated PR transcriptional activity in human breast cancer cells expressing altered cell cycle regulatory molecules.
Figures


References
-
- Alkarain, A., R. Jordan, and J. Slingerland. 2004. p27 deregulation in breast cancer: prognostic significance and implications for therapy. J. Mammary Gland Biol. Neoplasia 9:67-80. - PubMed
-
- Ansieau, S., E. Kowenz-Leutz, R. Dechend, and A. Leutz. 1997. B-Myb, a repressed trans-activating protein. J. Mol. Med. 75:815-819. - PubMed
-
- Bain, D. L., M. A. Franden, J. L. McManaman, G. S. Takimoto, and K. B. Horwitz. 2000. The N-terminal region of the human progesterone A-receptor. Structural analysis and the influence of the DNA binding domain. J. Biol. Chem. 275:7313-7320. - PubMed
-
- Bartkova, J., J. Lukas, H. Muller, D. Lutzhoft, M. Strauss, and J. Bartek. 1994. Cyclin D1 protein expression and function in human breast cancer. Int. J. Cancer 57:353-361. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
