A role for beta-actin in RNA polymerase III transcription
- PMID: 15574586
- PMCID: PMC535912
- DOI: 10.1101/gad.1250804
A role for beta-actin in RNA polymerase III transcription
Abstract
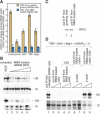
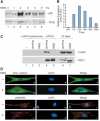
When transcription from the human U6 snRNA gene is reconstituted with recombinant factors and purified RNA polymerase III (pol III), pol III must be treated with CK2 to be active. We show that highly purified pol III contains associated beta-actin, and beta-actin localizes to an active U6 promoter in vivo. Pol III immunoprecipitated from IMR90 cells treated with a genotoxic agent lacks associated beta-actin and is inactive in the reconstituted assay. Transcription is regained upon treatment of pol III with CK2 and addition of beta-actin. This suggests that beta-actin associated with pol III is essential for basal pol III transcription.
Figures


References
-
- Ascough K.R. 2004. Endocytosis: Actin in the driving seat. Curr. Biol. 14: R124-R126. - PubMed
-
- Bettinger B.T., Gilbert, D.M., and Amberg, D.C. 2004. Actin up in the nucleus. Nat. Rev. Mol. Cell. Biol. 5: 410-415. - PubMed
-
- Cramer P., Bushnell, D.A., and Kornberg, R.D. 2001. Structural basis of transcription: RNA polymerase II at 2.8 angstrom resolution. Science 292: 1863-1876. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
