Nuclear calcium/calmodulin regulates memory consolidation
- PMID: 15574736
- PMCID: PMC6730218
- DOI: 10.1523/JNEUROSCI.1022-04.2004
Nuclear calcium/calmodulin regulates memory consolidation
Abstract
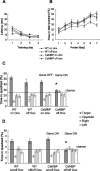
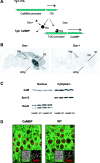
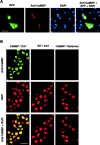
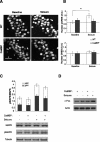
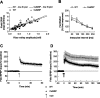
The neuronal response to a Ca2+ stimulus is a complex process involving direct Ca2+/calmodulin (CaM) actions as well as secondary activation of multiple signaling pathways such as cAMP and ERK (extracellular signal-regulated kinase). These signals can act in both the cytoplasm and the nucleus to control gene expression. To dissect the role of nuclear from cytoplasmic Ca2+/CaM signaling in memory formation, we generated transgenic mice that express a dominant inhibitor of Ca2+/CaM selectively in the nuclei of forebrain neurons and only after the animals reach adulthood. These mice showed diminished neuronal activity-induced phosphorylation of cAMP response element-binding protein, reduced expression of activity-induced genes, altered maximum levels of hippocampal long-term potentiation, and severely impaired formation of long-term, but not short-term, memory. Our results demonstrate that nuclear Ca2+/CaM signaling plays a critical role in memory consolidation in the mouse.
Figures
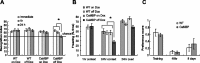
References
-
- Abel T, Nguyen PV, Barad M, Deuel TA, Kandel ER, Bourtchouladze R (1997) Genetic demonstration of a role for PKA in the late phase of LTP and in hippocampus-based long-term memory. Cell 88: 615-626. - PubMed
-
- Agell N, Aligue R, Alemany V, Castro A, Jaime M, Pujol MJ, Rius E, Serratosa J, Taules M, Bachs O (1998) New nuclear functions for calmodulin. Cell Calcium 23: 115-121. - PubMed
-
- Alarcon JM, Malleret G, Touzani K, Vronskaya S, Ishii S, Kandel ER, Barco A (2004) Chromatin acetylation, memory, and LTP are impaired in CBP+/- mice: a model for the cognitive deficit in Rubinstein-Taybi syndrome and its amelioration. Neuron 42: 947-959. - PubMed
-
- Bading H (2000) Transcription-dependent neuronal plasticity the nuclear calcium hypothesis. Eur J Biochem 267: 5280-5283. - PubMed
-
- Bourtchuladze R, Frenguelli B, Blendy J, Cioffi D, Schutz G, Silva AJ (1994) Deficient long-term memory in mice with a targeted mutation of the cAMP-responsive element-binding protein. Cell 79: 59-68. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous
