Synthetic genetic array analysis of the PtdIns 4-kinase Pik1p identifies components in a Golgi-specific Ypt31/rab-GTPase signaling pathway
- PMID: 15574876
- PMCID: PMC545911
- DOI: 10.1091/mbc.e04-08-0700
Synthetic genetic array analysis of the PtdIns 4-kinase Pik1p identifies components in a Golgi-specific Ypt31/rab-GTPase signaling pathway
Abstract
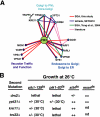
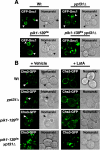
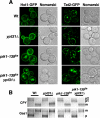
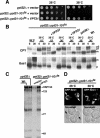
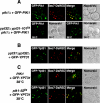
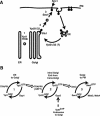
Phosphorylated derivatives of phosphatidylinositol are essential regulators of both endocytic and exocytic trafficking in eukaryotic cells. In Saccharomyces cerevisiae, the phosphatidylinositol 4-kinase, Pik1p generates a distinct pool of PtdIns(4)P that is required for normal Golgi structure and secretory function. Here, we utilize a synthetic genetic array analysis of a conditional pik1 mutant to identify candidate components of the Pik1p/PtdIns(4)P signaling pathway at the Golgi. Our data suggest a mechanistic involvement for Pik1p with a specific subset of Golgi-associated proteins, including the Ypt31p rab-GTPase and the TRAPPII protein complex, to regulate protein trafficking through the secretory pathway. We further demonstrate that TRAPPII specifically functions in a Ypt31p-dependent pathway and identify Gyp2p as the first biologically relevant GTPase activating protein for Ypt31p. We propose that multiple stage-specific signals, which may include Pik1p/PtdIns(4)P, TRAPPII and Gyp2p, impinge upon Ypt31 signaling to regulate Golgi secretory function.
Figures




Similar articles
-
Distinct roles for the yeast phosphatidylinositol 4-kinases, Stt4p and Pik1p, in secretion, cell growth, and organelle membrane dynamics.Mol Biol Cell. 2000 Aug;11(8):2673-89. doi: 10.1091/mbc.11.8.2673. Mol Biol Cell. 2000. PMID: 10930462 Free PMC article.
-
TRAPPII regulates exocytic Golgi exit by mediating nucleotide exchange on the Ypt31 ortholog RabERAB11.Proc Natl Acad Sci U S A. 2015 Apr 7;112(14):4346-51. doi: 10.1073/pnas.1419168112. Epub 2015 Mar 23. Proc Natl Acad Sci U S A. 2015. PMID: 25831508 Free PMC article.
-
Interaction of Pik1p and Sjl proteins in membrane trafficking.FEMS Yeast Res. 2005 Feb;5(4-5):363-71. doi: 10.1016/j.femsyr.2004.09.007. FEMS Yeast Res. 2005. PMID: 15691741
-
[Rab GTPases networks in membrane traffic in Saccharomyces cerevisiae].Yakugaku Zasshi. 2015;135(3):483-92. doi: 10.1248/yakushi.14-00246. Yakugaku Zasshi. 2015. PMID: 25759056 Review. Japanese.
-
Regulation of membrane traffic by Rab GEF and GAP cascades.Small GTPases. 2016 Oct;7(4):252-256. doi: 10.1080/21541248.2016.1213781. Epub 2016 Jul 18. Small GTPases. 2016. PMID: 27427966 Free PMC article. Review.
Cited by
-
The phosphatidylinositol 4,5-biphosphate and TORC2 binding proteins Slm1 and Slm2 function in sphingolipid regulation.Mol Cell Biol. 2006 Aug;26(15):5861-75. doi: 10.1128/MCB.02403-05. Mol Cell Biol. 2006. PMID: 16847337 Free PMC article.
-
The TRAPP complex: insights into its architecture and function.Traffic. 2008 Dec;9(12):2032-42. doi: 10.1111/j.1600-0854.2008.00833.x. Epub 2008 Oct 14. Traffic. 2008. PMID: 18801063 Free PMC article. Review.
-
Autophagy in the context of the cellular membrane-trafficking system: the enigma of Atg9 vesicles.Biochem Soc Trans. 2017 Dec 15;45(6):1323-1331. doi: 10.1042/BST20170128. Epub 2017 Nov 17. Biochem Soc Trans. 2017. PMID: 29150528 Free PMC article. Review.
-
TRAPP II complex assembly requires Trs33 or Trs65.Traffic. 2009 Dec;10(12):1831-44. doi: 10.1111/j.1600-0854.2009.00988.x. Epub 2009 Sep 14. Traffic. 2009. PMID: 19843283 Free PMC article.
-
Membrane-trafficking sorting hubs: cooperation between PI4P and small GTPases at the trans-Golgi network.Trends Cell Biol. 2011 Sep;21(9):515-25. doi: 10.1016/j.tcb.2011.05.005. Epub 2011 Jul 19. Trends Cell Biol. 2011. PMID: 21764313 Free PMC article. Review.
References
-
- Albert, S., and Gallwitz, D. (1999). Two new members of a family of Ypt/Rab GTPase activating proteins. J. Biol. Chem. 274, 33186-33189. - PubMed
-
- Audhya, A., and Emr, S. D. (2002). Stt4 PI4-kinase localizes to the plasma membrane and functions I the Pkc1-mediated MAP kinase cascade. Dev. Cell 2, 593-605. - PubMed
-
- Begley, M. J., Taylor, G. S., Kim, S.-A., Veine, D. M., Dixon, J. E., and Stuckey, J. A. (2003). Crystal structure of a phosphoinsitide phosphatase, MTMR 2, insights into myotubular myopathy and Charcot-Marie Tooth Syndrome. Mol. Cell 12, 1391-1402. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

