Cometabolism of a nongrowth substrate: L-serine utilization by Corynebacterium glutamicum
- PMID: 15574911
- PMCID: PMC535176
- DOI: 10.1128/AEM.70.12.7148-7155.2004
Cometabolism of a nongrowth substrate: L-serine utilization by Corynebacterium glutamicum
Abstract
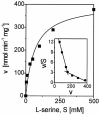
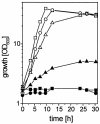
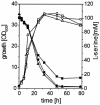
Despite its key position in central metabolism, L-serine does not support the growth of Corynebacterium glutamicum. Nevertheless, during growth on glucose, L-serine is consumed at rates up to 19.4 +/- 4.0 nmol min(-1) (mg [dry weight])(-1), resulting in the complete consumption of 100 mM L-serine in the presence of 100 mM glucose and an increased growth yield of about 20%. Use of 13C-labeled L-serine and analysis of cellularly derived metabolites by nuclear magnetic resonance spectroscopy revealed that the carbon skeleton of L-serine is mainly converted to pyruvate-derived metabolites such as L-alanine. The sdaA gene was identified in the genome of C. glutamicum, and overexpression of sdaA resulted in (i) functional L-serine dehydratase (L-SerDH) activity, and therefore conversion of L-serine to pyruvate, and (ii) growth of the recombinant strain on L-serine as the single substrate. In contrast, deletion of sdaA decreased the L-serine cometabolism rate with glucose by 47% but still resulted in degradation of L-serine to pyruvate. Cystathionine beta-lyase was additionally found to convert L-serine to pyruvate, and the respective metC gene was induced 2.4-fold under high internal L-serine concentrations. Upon sdaA overexpression, the growth rate on glucose is reduced 36% from that of the wild type, illustrating that even with glucose as a single substrate, intracellular L-serine conversion to pyruvate might occur, although probably the weak affinity of L-SerDH (apparent Km, 11 mM) prevents substantial L-serine degradation.
Figures


Similar articles
-
Metabolic engineering of Corynebacterium glutamicum for L-serine production.Appl Environ Microbiol. 2005 Nov;71(11):7139-44. doi: 10.1128/AEM.71.11.7139-7144.2005. Appl Environ Microbiol. 2005. PMID: 16269752 Free PMC article.
-
L-serine catabolism via an oxygen-labile L-serine dehydratase is essential for colonization of the avian gut by Campylobacter jejuni.Infect Immun. 2004 Jan;72(1):260-8. doi: 10.1128/IAI.72.1.260-268.2004. Infect Immun. 2004. PMID: 14688104 Free PMC article.
-
Impact of CO2/HCO3- Availability on Anaplerotic Flux in Pyruvate Dehydrogenase Complex-Deficient Corynebacterium glutamicum Strains.J Bacteriol. 2019 Sep 20;201(20):e00387-19. doi: 10.1128/JB.00387-19. Print 2019 Oct 15. J Bacteriol. 2019. PMID: 31358612 Free PMC article.
-
Rewiring the Central Metabolic Pathway for High-Yield l-Serine Production in Corynebacterium glutamicum by Using Glucose.Biotechnol J. 2019 Jun;14(6):e1800497. doi: 10.1002/biot.201800497. Epub 2019 May 20. Biotechnol J. 2019. PMID: 30791233
-
Effect of pyruvate dehydrogenase complex deficiency on L-lysine production with Corynebacterium glutamicum.Appl Microbiol Biotechnol. 2007 Sep;76(3):615-23. doi: 10.1007/s00253-007-0904-1. Epub 2007 Mar 2. Appl Microbiol Biotechnol. 2007. PMID: 17333167
Cited by
-
Metabolic Engineering of Corynebacterium glutamicum for Sustainable Production of the Aromatic Dicarboxylic Acid Dipicolinic Acid.Microorganisms. 2022 Mar 29;10(4):730. doi: 10.3390/microorganisms10040730. Microorganisms. 2022. PMID: 35456781 Free PMC article.
-
Identification and characterization of the dicarboxylate uptake system DccT in Corynebacterium glutamicum.J Bacteriol. 2008 Oct;190(19):6458-66. doi: 10.1128/JB.00780-08. Epub 2008 Jul 25. J Bacteriol. 2008. PMID: 18658264 Free PMC article.
-
Metabolic engineering of Escherichia coli for poly(3-hydroxybutyrate) production via threonine bypass.Microb Cell Fact. 2015 Nov 20;14:185. doi: 10.1186/s12934-015-0369-3. Microb Cell Fact. 2015. PMID: 26589676 Free PMC article.
-
Citrate utilization by Corynebacterium glutamicum is controlled by the CitAB two-component system through positive regulation of the citrate transport genes citH and tctCBA.J Bacteriol. 2009 Jun;191(12):3869-80. doi: 10.1128/JB.00113-09. Epub 2009 Apr 17. J Bacteriol. 2009. PMID: 19376865 Free PMC article.
-
Engineering of Serine-Deamination pathway, Entner-Doudoroff pathway and pyruvate dehydrogenase complex to improve poly(3-hydroxybutyrate) production in Escherichia coli.Microb Cell Fact. 2014 Dec 16;13:172. doi: 10.1186/s12934-014-0172-6. Microb Cell Fact. 2014. PMID: 25510247 Free PMC article.
References
-
- Awano, N., M. Wada, A. Kohdoh, T. Oikawa, H. Takagi, and S. Nakamori. 2003. Effect of cysteine desulfhydrase gene disruption on l-cysteine overproduction in Escherichia coli. Appl. Microbiol. Biotechnol. 62:239-243. - PubMed
-
- Brown, E. A., R. D'Ari, and E. B. Newman. 1990. A relationship between l-serine degradation and methionine biosynthesis in Escherichia coli K12. J. Gen. Microbiol. 136:1017-1023. - PubMed
-
- Cocaign, M., C. Monnet, and N. D. Lindley. 1993. Batch kinetics of Corynebacterium glutamicum during growth on various substrates: use of substrate mixtures to localize metabolic bottlenecks. Appl. Microbiol. Biotechnol. 40:526-530.
-
- Eggeling, L., and H. Sahm. 1999. l-Glutamate and l-lysine: traditional products with impetuous developments. Appl. Microbiol. Biotechnol. 52:146-153.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

