The Janus kinases (Jaks)
- PMID: 15575979
- PMCID: PMC545791
- DOI: 10.1186/gb-2004-5-12-253
The Janus kinases (Jaks)
Abstract
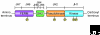
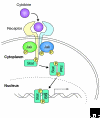
The Janus kinase (Jak) family is one of ten recognized families of non-receptor tyrosine kinases. Mammals have four members of this family, Jak1, Jak2, Jak3 and Tyrosine kinase 2 (Tyk2). Birds, fish and insects also have Jaks. Each protein has a kinase domain and a catalytically inactive pseudo-kinase domain, and they each bind cytokine receptors through amino-terminal FERM (Band-4.1, ezrin, radixin, moesin) domains. Upon binding of cytokines to their receptors, Jaks are activated and phosphorylate the receptors, creating docking sites for signaling molecules, especially members of the signal transducer and activator of transcription (Stat) family. Mutations of the Drosophila Jak (Hopscotch) have revealed developmental defects, and constitutive activation of Jaks in flies and humans is associated with leukemia-like syndromes. Through the generation of Jak-deficient cell lines and gene-targeted mice, the essential, nonredundant functions of Jaks in cytokine signaling have been established. Importantly, deficiency of Jak3 is the basis of human autosomal recessive severe combined immunodeficiency (SCID); accordingly, a selective Jak3 inhibitor has been developed, forming a new class of immunosuppressive drugs.
Figures
References
-
- Firmbach-Kraft I, Byers M, Shows T, Dalla-Favera R, Krolewski JJ. Tyk2, prototype of a novel class of non-receptor tyrosine kinase genes. Oncogene. 1990;5:1329–1336. This paper and [2-6] were the first studies to report the cloning of Jaks. - PubMed
-
- Wilks AF. Cloning members of protein-tyrosine kinase family using polymerase chain reaction. Methods Enzymol. 1991;200:533–546. See [1] - PubMed
-
- Harpur AG, Andres AC, Ziemiecki A, Aston RR, Wilks AF. JAK2, a third member of the JAK family of protein tyrosine kinases. Oncogene. 1992;7:1347–1353. See [1] - PubMed
-
- Krolewski JJ, Lee R, Eddy R, Shows TB, Dalla-Favera R. Identification and chromosomal mapping of new human tyrosine kinase genes. Oncogene. 1990;5:277–282. See [1] - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

