Mapping the functional domains of the Golgi stacking factor GRASP65
- PMID: 15576368
- PMCID: PMC4443495
- DOI: 10.1074/jbc.M412407200
Mapping the functional domains of the Golgi stacking factor GRASP65
Abstract
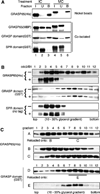
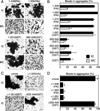
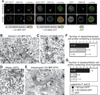
The Golgi reassembly stacking protein (GRASP) family has been implicated in the stacking of Golgi cisternae and the regulation of Golgi disassembly/reassembly during mitosis in mammalian cells. GRASP65 is a dimer that can directly link adjacent surfaces through trans-oligomerization in a mitotically regulated manner. Here we show that the N-terminal GRASP domain (amino acids 1-201) is both necessary and sufficient for dimerization and trans-oligomerization but is not mitotically regulated. The C-terminal serine/proline-rich domain (amino acids 202-446) cannot dimerize nor can it link adjacent surfaces. It does, however, confer mitotic regulation on the GRASP domain through multiple sites phosphorylated by the mitotic kinases, cdc2/B1, and the polo-like kinase. Transient expression corroborated these results by showing that the GRASP domain alone inhibited mitotic fragmentation of the Golgi apparatus.
Figures



References
-
- Rambourg A, Clermont Y. In: The Golgi Apparatus. Berger EG, Roth J, editors. Switzerland: Birkhauser Verlag, Basel; 1997. pp. 37–61.
-
- Barr FA, Puype M, Vandekerckhove J, Warren G. Cell. 1997;91:253–262. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

