Ser95, Asn97, and Thr78 are important for the catalytic function of porcine NADP-dependent isocitrate dehydrogenase
- PMID: 15576556
- PMCID: PMC2253315
- DOI: 10.1110/ps.041091805
Ser95, Asn97, and Thr78 are important for the catalytic function of porcine NADP-dependent isocitrate dehydrogenase
Abstract
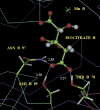
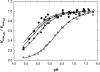
The mammalian mitochondrial NADP-dependent isocitrate dehydrogenase is a citric acid cycle enzyme and an important contributor to cellular defense against oxidative stress. The Mn(2+)-isocitrate complex of the porcine enzyme was recently crystallized; its structure indicates that Ser(95), Asn(97), and Thr(78) are within hydrogen-bonding distance of the gamma-carboxylate of enzyme-bound isocitrate. We used site-directed mutagenesis to replace each of these residues by Ala and Asp. The wild-type and mutant enzymes were expressed in Escherichia coli and purified to homogeneity. All the enzymes retain their native dimeric structures and secondary structures as monitored by native gel electrophoresis and circular dichroism, respectively. V(max) of the three alanine mutants is decreased to 24%-38% that of wild-type enzyme, with further decreases in the aspartate mutants. For T78A and S95A mutants, the major changes are the 10- to 100-fold increase in the K(m) values for isocitrate and Mn(2+). The results suggest that Thr(78) and Ser(95) function to strengthen the enzyme's affinity for Mn(2+)-isocitrate by hydrogen bonding to the gamma-carboxylate of isocitrate. For the Asn(97) mutants, the K(m) values are much less affected. The major change in the N97A mutant is the increase in pK(a) of the ionizable metal-liganded hydroxyl of enzyme-bound isocitrate from 5.23 in wild type to 6.23 in the mutant enzyme. The hydrogen bond between Asn(97) and the gamma-carboxylate of isocitrate may position the substrate to promote a favorable lowering of the pK of the enzyme-isocitrate complex. Thus, Thr(78), Ser(95), and Asn(97) perform important but distinguishable roles in catalysis by porcine NADP-specific isocitrate dehydrogenase.
Figures


Similar articles
-
Ligands of the Mn2+ bound to porcine mitochondrial NADP-dependent isocitrate dehydrogenase, as assessed by mutagenesis.Biochemistry. 2004 Mar 16;43(10):2821-8. doi: 10.1021/bi030253f. Biochemistry. 2004. PMID: 15005617
-
Evaluation by site-directed mutagenesis of aspartic acid residues in the metal site of pig heart NADP-dependent isocitrate dehydrogenase.Biochemistry. 2000 Mar 7;39(9):2193-200. doi: 10.1021/bi9919753. Biochemistry. 2000. PMID: 10694384
-
Identification by mutagenesis of arginines in the substrate binding site of the porcine NADP-dependent isocitrate dehydrogenase.J Biol Chem. 2000 Feb 25;275(8):5606-12. doi: 10.1074/jbc.275.8.5606. J Biol Chem. 2000. PMID: 10681542
-
Evaluation by mutagenesis of the roles of His309, His315, and His319 in the coenzyme site of pig heart NADP-dependent isocitrate dehydrogenase.Biochemistry. 2002 Apr 30;41(17):5637-43. doi: 10.1021/bi0200716. Biochemistry. 2002. PMID: 11969425
-
Location of the coenzyme binding site in the porcine mitochondrial NADP-dependent isocitrate dehydrogenase.J Biol Chem. 2005 Aug 26;280(34):30349-53. doi: 10.1074/jbc.M505828200. Epub 2005 Jun 23. J Biol Chem. 2005. PMID: 15975917
Cited by
-
The complex structures of isocitrate dehydrogenase from Clostridium thermocellum and Desulfotalea psychrophila suggest a new active site locking mechanism.FEBS Open Bio. 2012 Jul 7;2:159-72. doi: 10.1016/j.fob.2012.06.003. Print 2012. FEBS Open Bio. 2012. PMID: 23650595 Free PMC article.
-
Dual compartmental localization and function of mammalian NADP+-specific isocitrate dehydrogenase in yeast.Arch Biochem Biophys. 2008 Apr 1;472(1):17-25. doi: 10.1016/j.abb.2008.01.025. Epub 2008 Feb 6. Arch Biochem Biophys. 2008. PMID: 18275837 Free PMC article.
References
-
- Bailey, J.M. and Colman, R.F. 1985. Affinity labeling of NADP+-specific isocitrate dehydrogenase by a new fluorescent nucleotide analog, 2-[(4-bromo-2,3-dioxobutyl)thio]-1,N6-ethenoadenosine 2′,5′-bisphosphate. Biochemistry 24 5367–5377. - PubMed
-
- Benderdour, M., Charron, G., deBlois, D., Comte, B., and Des Rosiers, C. 2003. Cardiac mitochondrial NADP+-isocitrate dehydrogenase is inactivated through 4-hydroxynonenal adduct formation: An event that precedes hypertrophy development. J. Biol. Chem. 278 45154–45159. - PubMed
-
- Bradford, M.M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72 248–254. - PubMed
-
- Ceccarelli, C., Grodsky, N.B., Ariyaratne, N., Colman, R.F., and Bahnson, B.J. 2002. Crystal structure of porcine mitochondrial NADP+-dependent isocitrate dehydrogenase complexed with Mn2+ and isocitrate. Insights into the enzyme mechanism. J. Biol. Chem. 277 43454–43462. - PubMed
-
- Colman, R.F. 1972. Role of metal ions in reactions catalyzed by pig heart triphosphopyridine nucleotide-dependent isocitrate dehydrogenase. II. Effect on catalytic properties and reactivity of amino acid residues. J. Biol. Chem. 247 215–223. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

