PriA helicase and SSB interact physically and functionally
- PMID: 15576682
- PMCID: PMC535688
- DOI: 10.1093/nar/gkh980
PriA helicase and SSB interact physically and functionally
Abstract
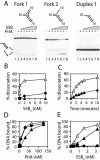
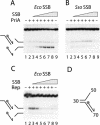
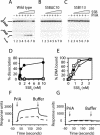
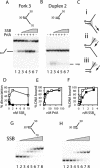
PriA helicase is the major DNA replication restart initiator in Escherichia coli and acts to reload the replicative helicase DnaB back onto the chromosome at repaired replication forks and D-loops formed by recombination. We have discovered that PriA-catalysed unwinding of branched DNA substrates is stimulated specifically by contact with the single-strand DNA binding protein of E.coli, SSB. This stimulation requires binding of SSB to the initial DNA substrate and is effected via a physical interaction between PriA and the C-terminus of SSB. Stimulation of PriA by the SSB C-terminus may act to ensure that efficient PriA-catalysed reloading of DnaB occurs only onto the lagging strand template of repaired forks and D-loops. Correlation between the DNA repair and recombination defects of strains harbouring an SSB C-terminal mutation with inhibition of this SSB-PriA interaction in vitro suggests that SSB plays a critical role in facilitating PriA-directed replication restart. Taken together with previous data, these findings indicate that protein-protein interactions involving SSB may coordinate replication fork reloading from start to finish.
Figures

References
-
- Lindahl T. (1996) The Croonian Lecture, 1996: endogenous damage to DNA. Philos. Trans. R. Soc. Lond. B Biol. Sci., 351, 1529–1538. - PubMed
-
- Vilette D., Ehrlich,S.D. and Michel,B. (1995) Transcription-induced deletions in Escherichia coli plasmids. Mol. Microbiol., 17, 493–504. - PubMed
-
- McGlynn P. and Lloyd,R.G. (2000) Modulation of RNA polymerase by (p)ppGpp reveals a RecG-dependent mechanism for replication fork progression. Cell, 101, 35–45. - PubMed
-
- Huertas P. and Aguilera,A. (2003) Cotranscriptionally formed DNA:RNA hybrids mediate transcription elongation impairment and transcription-associated recombination. Mol. Cell, 12, 711–721. - PubMed
-
- Cox M.M., Goodman,M.F., Kreuzer,K.N., Sherratt,D.J., Sandler,S.J. and Marians,K.J. (2000) The importance of repairing stalled replication forks. Nature, 404, 37–41. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

