TcpH influences virulence gene expression in Vibrio cholerae by inhibiting degradation of the transcription activator TcpP
- PMID: 15576780
- PMCID: PMC532408
- DOI: 10.1128/JB.186.24.8309-8316.2004
TcpH influences virulence gene expression in Vibrio cholerae by inhibiting degradation of the transcription activator TcpP
Abstract
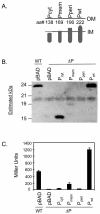
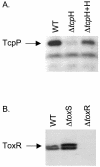
Expression of toxT, the transcription activator of cholera toxin and pilus production in Vibrio cholerae, is the consequence of a complex cascade of regulatory events that culminates in activation of the toxT promoter by TcpP and ToxR, two membrane-localized transcription factors. Both are encoded in operons with genes whose products, TcpH and ToxS, which are also membrane localized, are hypothesized to control their activity. In this study we analyzed the role of TcpH in controlling TcpP function. We show that a mutant of V. cholerae lacking TcpH expressed virtually undetectable levels of TcpP, although tcpP mRNA levels remain unaffected. A time course experiment showed that levels of TcpP, expressed from a plasmid, are dramatically reduced over time without co-overexpression of TcpH. By contrast, deletion of toxS did not affect ToxR protein levels. A fusion protein in which the TcpP periplasmic domain is replaced with that of ToxR remains stable, suggesting that the periplasmic domain of TcpP is the target for degradation of the protein. Placement of the periplasmic domain of TcpP on ToxR, an otherwise stable protein, results in instability, providing further evidence for the hypothesis that the periplasmic domain of TcpP is a target for degradation. Consistent with this interpretation is our finding that derivatives of TcpP lacking a periplasmic domain are more stable in V. cholerae than are derivatives in which the periplasmic domain has been truncated. This work identifies at least one role for the periplasmic domain of TcpP, i.e., to act as a target for a protein degradation pathway that regulates TcpP levels. It also provides a rationale for why the V. cholerae tcpH mutant strain is avirulent. We hypothesize that regulator degradation may be an important mechanism for regulating virulence gene expression in V. cholerae.
Figures



Similar articles
-
Formation of an Intramolecular Periplasmic Disulfide Bond in TcpP Protects TcpP and TcpH from Degradation in Vibrio cholerae.J Bacteriol. 2015 Nov 16;198(3):498-509. doi: 10.1128/JB.00338-15. Print 2016 Feb 1. J Bacteriol. 2015. PMID: 26574510 Free PMC article.
-
Regulated intramembrane proteolysis of the virulence activator TcpP in Vibrio cholerae is initiated by the tail-specific protease (Tsp).Mol Microbiol. 2015 Sep;97(5):822-31. doi: 10.1111/mmi.13069. Epub 2015 Jul 30. Mol Microbiol. 2015. PMID: 25999037
-
A new level in the Vibrio cholerae ToxR virulence cascade: AphA is required for transcriptional activation of the tcpPH operon.Mol Microbiol. 1999 Feb;31(3):763-71. doi: 10.1046/j.1365-2958.1999.01215.x. Mol Microbiol. 1999. PMID: 10048021
-
Co-ordinate expression of virulence genes by ToxR in Vibrio cholerae.Mol Microbiol. 1992 Feb;6(4):451-8. doi: 10.1111/j.1365-2958.1992.tb01489.x. Mol Microbiol. 1992. PMID: 1560773 Review.
-
Expression of Vibrio cholerae virulence genes in response to environmental signals.Curr Issues Intest Microbiol. 2002 Sep;3(2):29-38. Curr Issues Intest Microbiol. 2002. PMID: 12400636 Review.
Cited by
-
Cyclic diguanylate regulates Vibrio cholerae virulence gene expression.Infect Immun. 2005 Sep;73(9):5873-82. doi: 10.1128/IAI.73.9.5873-5882.2005. Infect Immun. 2005. PMID: 16113306 Free PMC article.
-
A fadD mutant of Vibrio cholerae is impaired in the production of virulence factors and membrane localization of the virulence regulatory protein TcpP.Infect Immun. 2011 Jan;79(1):258-66. doi: 10.1128/IAI.00663-10. Epub 2010 Nov 1. Infect Immun. 2011. PMID: 21041490 Free PMC article.
-
The membrane-bound transcriptional regulator CadC is activated by proteolytic cleavage in response to acid stress.J Bacteriol. 2008 Jul;190(14):5120-6. doi: 10.1128/JB.00012-08. Epub 2008 May 16. J Bacteriol. 2008. PMID: 18487329 Free PMC article.
-
Transmembrane Transcription Regulators Are Widespread in Bacteria and Archaea.Microbiol Spectr. 2023 Jun 15;11(3):e0026623. doi: 10.1128/spectrum.00266-23. Epub 2023 May 8. Microbiol Spectr. 2023. PMID: 37154724 Free PMC article.
-
Bile Salts Promote ToxR Regulon Activation during Growth under Virulence-Inducing Conditions.Infect Immun. 2021 Nov 16;89(12):e0044121. doi: 10.1128/IAI.00441-21. Epub 2021 Sep 20. Infect Immun. 2021. PMID: 34543121 Free PMC article.
References
-
- Bothmann, H., and A. Pluckthun. 1998. Selection for a periplasmic factor improving phage display and functional periplasmic expression. Nat. Biotechnol. 16:376-380. - PubMed
-
- Carroll, P. A., K. T. Tashima, M. B. Rogers, V. J. DiRita, and S. B. Calderwood. 1997. Phase variation in tcpH modulates expression of the ToxR regulon in Vibrio cholerae. Mol. Microbiol. 25:1099-1111. - PubMed
-
- Chen, R., and U. Henning. 1996. A periplasmic protein (Skp) of Escherichia coli selectively binds a class of outer membrane proteins. Mol. Microbiol. 19:1287-1294. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

