A phospholipid sensor controls mechanogating of the K+ channel TREK-1
- PMID: 15577940
- PMCID: PMC544907
- DOI: 10.1038/sj.emboj.7600494
A phospholipid sensor controls mechanogating of the K+ channel TREK-1
Abstract
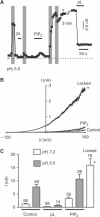
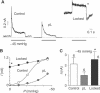
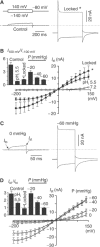
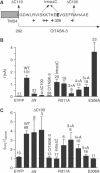
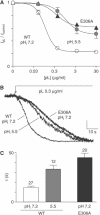
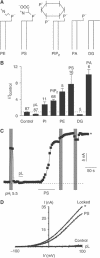
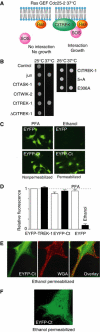
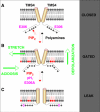
TREK-1 (KCNK2 or K(2P)2.1) is a mechanosensitive K(2P) channel that is opened by membrane stretch as well as cell swelling. Here, we demonstrate that membrane phospholipids, including PIP(2), control channel gating and transform TREK-1 into a leak K(+) conductance. A carboxy-terminal positively charged cluster is the phospholipid-sensing domain that interacts with the plasma membrane. This region also encompasses the proton sensor E306 that is required for activation of TREK-1 by cytosolic acidosis. Protonation of E306 drastically tightens channel-phospholipid interaction and leads to TREK-1 opening at atmospheric pressure. The TREK-1-phospholipid interaction is critical for channel mechano-, pH(i)- and voltage-dependent gating.
Figures

References
-
- Aronheim A (2001) Ras signaling pathway for analysis of protein–protein interactions. Methods Enzymol 332: 260–270 - PubMed
-
- Bang H, Kim Y, Kim D (2000) TREK-2, a new member of the mechanosensitive tandem pore K+ channel family. J Biol Chem 275: 17412–17419 - PubMed
-
- Baukrowitz T, Schulte U, Oliver D, Herlitze S, Krauter T, Tucker SJ, Ruppersberg JP, Fakler B (1998) PIP2 and PIP as determinants for ATP inhibition of KATP channels. Science 282: 1141–1144 - PubMed
-
- Bockenhauer D, Zilberberg N, Goldstein SA (2001) KCNK2: reversible conversion of a hippocampal potassium leak into a voltage-dependent channel. Nat Neurosci 4: 486–491 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
